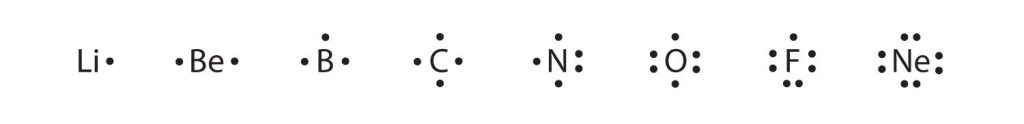Home » Student Resources » Online Chemistry Textbooks » CH105: Consumer Chemistry » CH105: Chapter 3 – Ionic and Covelent Bonding
MenuCH105: Consumer Chemistry
Chapter 3 – Ionic and Covalent Bonding
This content can also be downloaded as a PDF file. For the interactive PDF, adobe reader is required for full functionality.
This text is published under creative commons licensing, for referencing and adaptation, please click here.
Sections:
3.1 Two Types of Bonding
3.2 Ions
Electron Transfer
Lewis Diagrams
3.3 Covalent Bonding and Simple Molecular Compounds
Covalent Bonds
Electron Sharing
Covalent Bonds Between Different Atoms
Multiple Covalent Bonds
3.4 Chapter Summary
Ionic Compounds
Covalent Compounds
3.5 References
There are only 118 known chemical elements but tens of millions of known chemical compounds. Compounds can be very complex combinations of atoms, but many important compounds are fairly simple. Table salt, as we have seen, consists of only two elements: sodium and chlorine. Nevertheless, the compound has properties completely different from either elemental sodium (a chemically reactive metal) or elemental chlorine (a poisonous, green gas). We will see additional examples of such differences in section 3.3 of this chapter covering “Covalent Bonding and Simple Molecular Compounds”, as we consider how atoms combine to form compounds.
3.1 Two Types of Bonding
Atoms can join together by forming a chemical bond, which is a very strong attraction between two atoms. Chemical bonds are formed when electrons in different atoms interact with each other to make an arrangement that is more stable than when the atoms are apart.
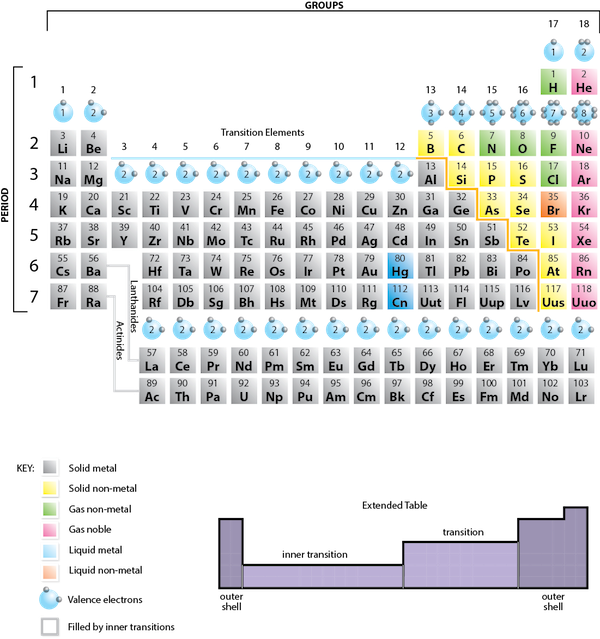
What causes atoms to make a chemical bond with other atoms, rather than remaining as individual atoms? A clue comes by considering the noble gas elements, the rightmost column of the periodic table. These elements—helium, neon, argon, krypton, xenon, and radon—do not form compounds very easily, which suggests that they are especially stable as lone atoms. What else do the noble gas elements have in common? Except for helium, they all have eight valence electrons. Chemists have concluded that atoms are especially stable if they have eight electrons in their outermost shell. This useful rule of thumb is called the octet rule, and it is a key to understanding why compounds form.
There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. One way is the transfer of electrons between two atoms until both atoms have octets. Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but with the transfer of electrons the individual atoms acquire a nonzero electric charge. Those that lose electrons become positively charged, and those that gain electrons become negatively charged. Charged atoms are called ions. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds.
The second way for an atom to obtain an octet of electrons is by sharing electrons with another atom. These shared electrons simultaneously occupy the outermost shell of both atoms. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Section 4 “Covalent Bonding and Simple Molecular Compounds”.
3.2 Ions
Most atoms do not have eight electrons in their valence electron shell. Some atoms have only a few electrons in their outer shell, while some atoms lack only one or two electrons to have an octet. In cases where an atom has three or fewer valence electrons, the atom may lose those valence electrons quite easily until what remains is an octet in the next lower shell. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons in the nucleus. Positively charged ions are called cations. Most metals become cations when they make ionic compounds.
Some atoms have nearly eight electrons in their valence shell and can readily gain additional valence electrons until they have an octet. When these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Negatively charged ions are called anions. Most nonmetals become anions when they make ionic compounds.
Electron Transfer
We can use valence electrons and the octet rule to illustrate the electron transfer process between sodium atoms and chlorine atoms.
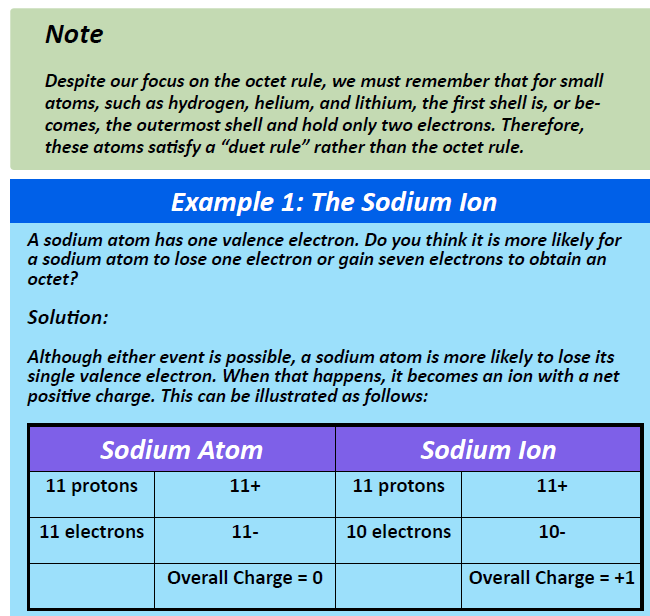
As demonstrated in Example 1 (below), sodium is likely to achieve an octet in its outermost shell by losing its one valence electron.
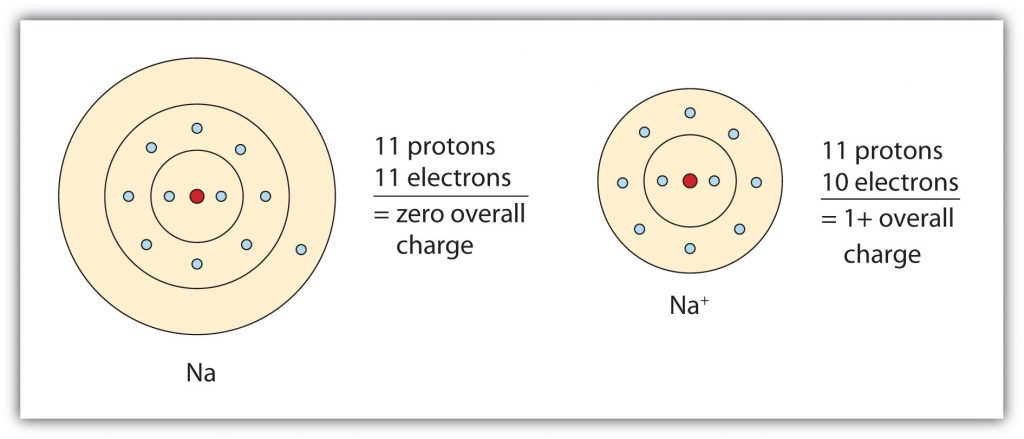
The cation produced in this way, Na+, is called the sodium ion to distinguish it from the element. The outermost shell of the sodium ion is the second electron shell, which has eight electrons in it. The octet rule has been satisfied. Figure 3.1 “The Formation of a Sodium Ion” is a graphical depiction of this process.
Fig 3.1. The Formation of a Sodium Ion. On the left, a sodium atom has 11 electrons. On the right, the sodium ion only has 10 electrons and a 1+ charge. Note that the sodium ion now has an outer electron shell that has eight electrons, fulfilling the octet rule.
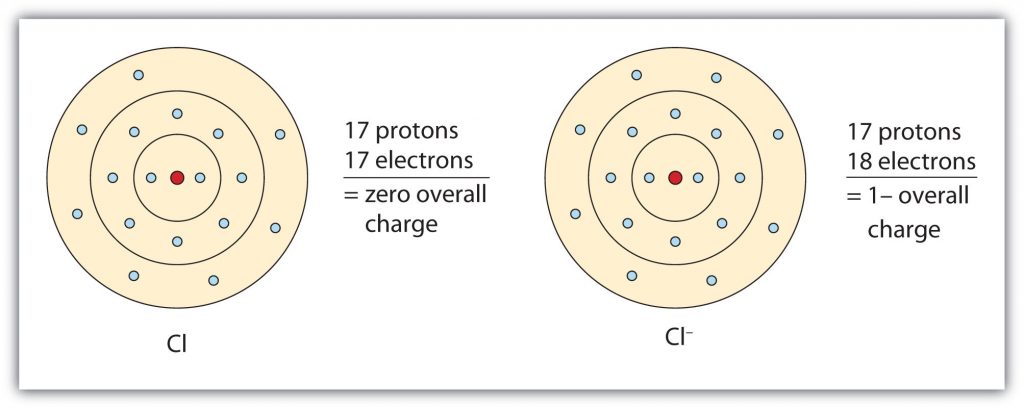
For a chlorine atom only one more electron is needed to achieve an octet in chlorine’s valence shell, because it has seven electrons in its outermost shell (Fig 3.2). In table salt, NaCl, this electron comes from the sodium atom.
Fig 3.2. The Formation of a Chloride Ion. On the left, a chlorine atom has 17 electrons. On the right, the chloride ion has gained an extra electron for a total of 18 electrons and a 1– charge. Note that the chloride ion has now filled its outer shell and contains eight electrons, satisfying the octet rule.
In this case, the ion has the same outermost shell as the original atom, but now that shell has eight electrons in it. Once again, the octet rule has been satisfied. The resulting anion, Cl−, is called the chloride ion; note the slight change in the suffix (-ide instead of -ine) to create the name of this anion. Figure 3.2 “The Formation of a Chlorine Ion” is a graphical depiction of this process.
With two oppositely charged ions, there is an electrostatic attraction between them because opposite charges attract. The resulting combination is the ionic compound sodium chloride. Notice that there are no leftover electrons. The number of electrons lost by the sodium atom (one) equals the number of electrons gained by the chlorine atom (one), so the compound is electrically neutral. In macroscopic samples of sodium chloride, there are billions and billions of sodium and chloride ions, although there is always the same number of cations and anions.
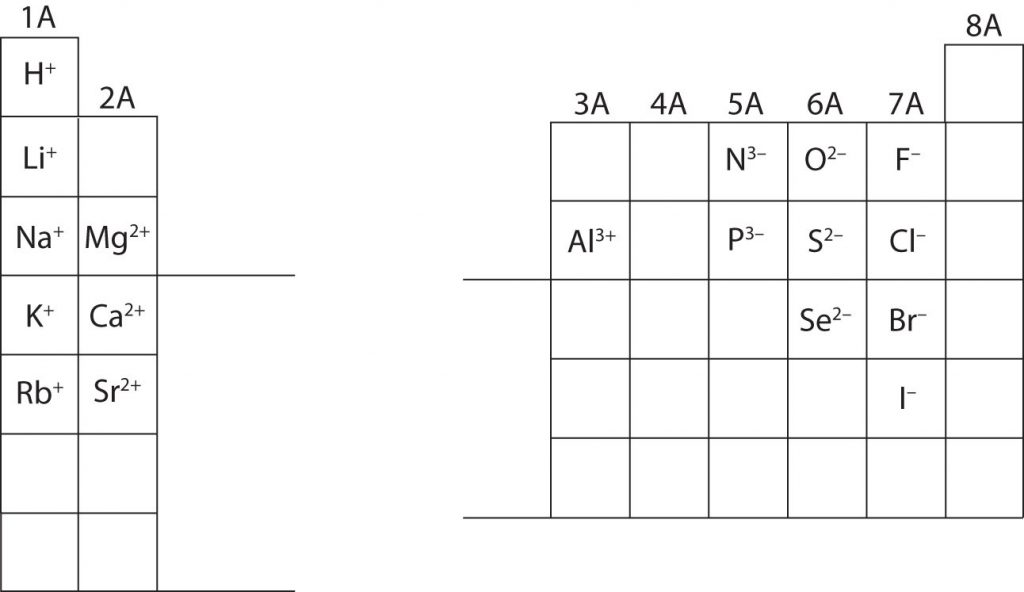
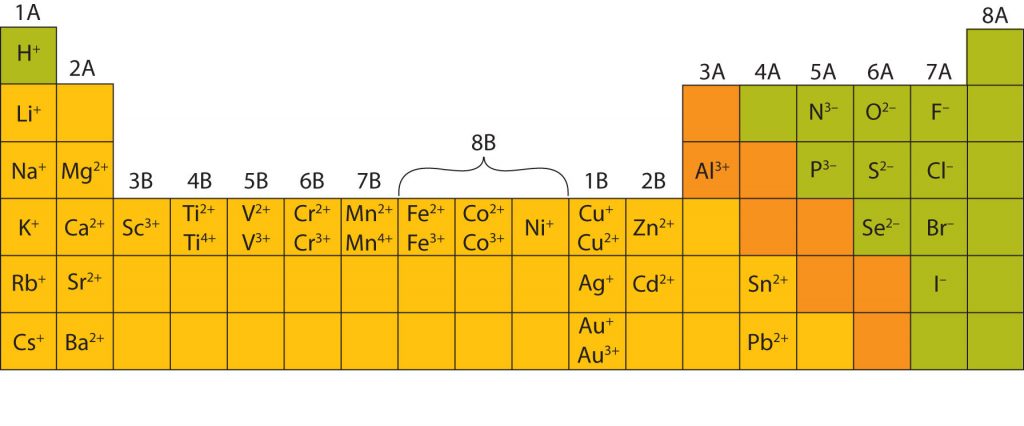
In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge, because they have the same number of valence electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions. For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table in the family 7A column, the halogens form ions having a 1− charge. Figure 3.3 “Predicting Ionic Charges” shows how the charge on many ions can be predicted by the location of an element on the periodic table. Note the convention of first writing the number and then the sign on a multiply-charged ion. The barium cation is written Ba2+ not Ba+2.
Fig 3.3. Predicting Ionic Charge. The Octet Rule can be used to help you predict how many electrons an element must gain or lose to achieve an electron configuration similar to the Noble Gases. Note that the first 3 Main Group Columns typically lose electrons to achieve the octet, while columns 5-7 typically gain electrons to reach the octet. Notice column 4, it is right in the middle and is left blank. Elements in column 4 contain 4 electrons in their valence shell. Thus, they would either need to gain 4 e- or lose 4- to reach the octet state. However, a 4 e- gain or loss is too much charge for one atom to easily hold and typically becomes unstable, unless the atom is very large! (Lead for example can lose 4 e-). For most elements in row 4, they tend to share electrons in covalent bonds (described in section 4), rather than gaining or losing electrons to form ionic bonds.
Lewis Diagrams
Chemists use simple diagrams to show an atom’s valence electrons and how they transfer. These diagrams have two advantages over the electron shell diagrams introduced in Chapter 2 “Elements, Atoms, and the Periodic Table”. First, they show only valence electrons. Second, instead of having a circle around the chemical symbol to represent the electron shell, they have up to eight dots around the symbol; each dot represents a valence electron. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. For example, the representation for sodium is as follows:
and the representation for chlorine is as follows:
These diagrams are called Lewis electron dot diagrams, or simply Lewis diagrams, after Gilbert N. Lewis, the American chemist who introduced them. Figure 3.4 “Lewis Diagrams of the Elements Lithium through Neon” shows the Lewis diagrams of the elements lithium through neon, which is the entire second period of the periodic table. For the main group elements, the number of valence electrons is the same as the group number listed at the top of the periodic table.
Fig 3.4. Lewis Diagrams of the Elements Lithium through Neon
The transfer of electrons can be illustrated easily with Lewis diagrams. In the following diagram sodium transfers an electron to chlorine:
In representing the final formula, the dots are omitted.
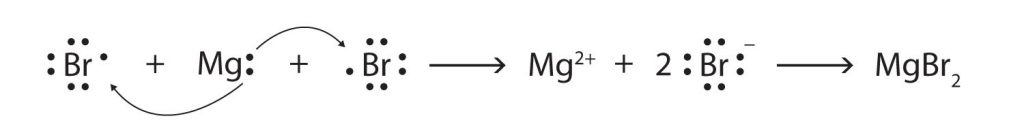
Some ionic compounds have different numbers of cations and anions. In those cases, electron transfer occurs between more than one atom. The following diagram shows the formation of magnesium bromide from two bromine atoms and one magnesium atom:
Most of the elements in ionic compounds form an ion that has a characteristic charge. For example, sodium makes ionic compounds in which the sodium ion always has a 1+ charge. Chlorine makes ionic compounds in which the chloride ion always has a 1− charge. Some elements, especially transition metals, can form ions with different charges. Figure 3.5 “Charges of the Monatomic Ions” shows the characteristic charges for some of these ions. As we saw in Figure 3.1 “The Formation of a Sodium Ion”, there is a pattern to the charges on many of the main group ions, but there is no simple pattern for transition metal ions (or for the larger main group elements).
Fig 3.5. Charges of Monoatomic Ions. Note that some atoms, especially transition metals, commonly form ions of different charges. This is due to internal orbital subshells that are very close to the valence shell and can be used in bond formation.
Overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an overall neutral net charge. Of note, ionic bonds usually occur between a metal and a nonmetal. The compound formulas are written with the cation first followed by the anion, and the lowest ratio of cations and anions are used to create a net neutral compound. Bonds that occur between nonmetals with other nonmetals, or nonmetals with semi-metals (or metalloids as they are also called), use covalent bonding, or the sharing of electrons, which is the topic of Section 3.3, below.
3.3 Covalent Bonding and Simple Molecular Compounds
Sections 3.1 and 3.2 discussed ionic bonding, which results from the transfer of electrons among atoms or groups of atoms. In this section, we will consider another type of bonding—covalent bonding. We will examine how atoms share electrons to form these bonds, and we will begin to explore how the resulting compounds, such as cholesterol, are different from ionic compounds.
Covalent Bonds
You have already seen examples of substances that contain covalent bonds. One substance was carbon dioxide (CO2). You can tell from its formula that it is not an ionic compound; it is not composed of a metal and a nonmetal. Consequently, its properties are different from those of ionic compounds.
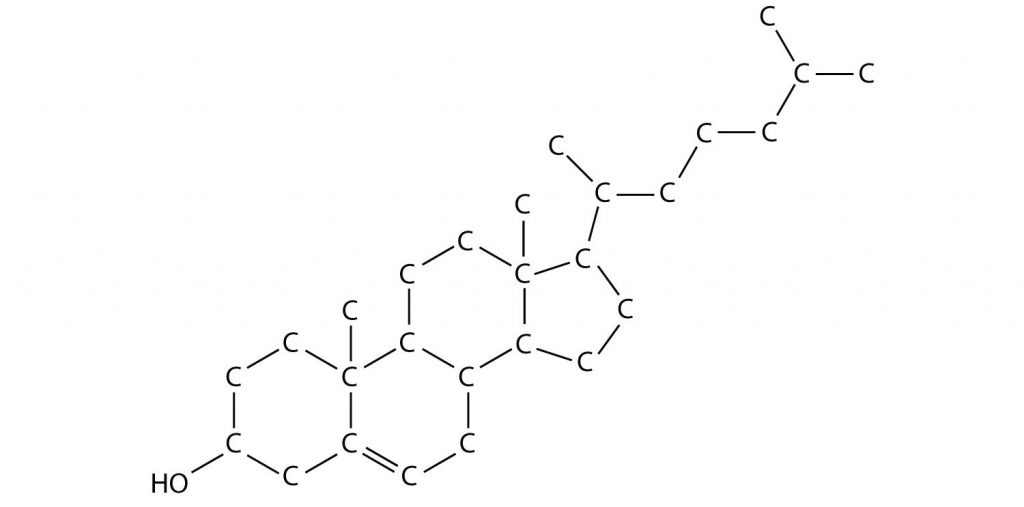
Figure 3.6: A Molecular Model of Cholesterol The structure above shows a molecular model of cholesterol. Note that the hydrogen atoms that are bonded to carbons within the molecule are not shown to save space. In Chapter 5, you will learn more about common shorthand techniques to draw complex organic molecules, like cholesterol.
Electron Sharing
Sections 3.1 and 3.2 described how electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell. Because most filled electron shells have eight electrons in them, chemists called this tendency the octet rule. But there is another way an atom can achieve a full valence shell: atoms can share electrons to reach the octet state (or the duet state in the case of hydrogen).
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) We can represent the two individual hydrogen atoms as follows:
In this situation neither hydrogen can reach the preferred duet state. In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound.
Chemists frequently use Lewis diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
The Lewis diagram of two hydrogen atoms sharing electrons looks like this:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
Remember that the dash, also referred to as a single bond, represents a pair of bonding electrons.
The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10−11 m, or 74 picometers (pm; 1 pm = 1 × 10−12 m). This particular bond length represents a balance between several forces: the attractions between oppositely charged electrons and nuclei, the repulsion between two negatively charged electrons, and the repulsion between two positively charged nuclei. If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following electron dot diagrams:
Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule:
The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Covalent Bonds Between Different Atoms
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:
Each atom needs one additional electron to complete its valence shell. By each contributing one electron, they make the following molecule:
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the valence electron shells are filled for both atoms.
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH4), with one carbon atom and four hydrogen atoms, can be represented as follows:
Atoms typically form a characteristic number of covalent bonds in compounds. Figure 3.7 “Periodic Table with Lewis Structures” shows valence electron configurations of each element family (or column).
Fig 3.7 Periodic Table with Lewis Structures. Each family shows a representative lewis structure for that group of elements. For the nonmetals (Families 4A, 5A, 6A, and 7A) they can accept a complementary number of shared bonds to reach the octet state. Family 4A can share 4 covalent bonds (4 + 4 = 8), whereas Families 5A, 6A, and 7A can share 3, 2, and 1 covalent bond(s), respectively, to achieve the octet state. Exceptions to the octet rule do exist. For example, hydrogen can be considered to be in Group 1 or Group 7A because it has properties similar to both groups. Hydrogen can participate in either ionic or covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has one electron to start with, it can only make one covalent bond. Similarly, boron has 3 electrons in its outer shell. This nonmetal typically forms 3 covalent bonds, having a maximum of 6 electrons in its outer shell. Thus, boron can never reach the octet state. Other atoms can have expanded orbitals and accept additional covalent bonds. Two of these that are important for living systems is sulfur and phosphorus. By the octet rule, sulfur can make 2 covalent bonds and phosphorus 3 covalent bonds. Sulfur can also have expanded orbitals to accept 4 or 6 covalent bonds, and phosphorus can expand to 5 covalent bonds.
Source: Exploring Our Fluid Earth, a product of the Curriculum Research & Development Group (CRDG), College of Education. © University of Hawaii, 2017. Available at: https://manoa.hawaii.edu/exploringourfluidearth/chemical/chemistry-and-seawater/covalent-bonding
Multiple Covalent Bonds
In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares two electrons. Consider carbon dioxide (CO2). If each oxygen atom shares one electron with the carbon atom, we get the following:
This does not give either the carbon or oxygen atoms a complete octet; The carbon atom only has six electrons in its valence shell and each oxygen atom only has seven electrons in its valence shell. Thus, none of the atoms can reach the octet state in the current configuration. As written, this would be an unstable molecular conformation.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C2H2), whose Lewis diagram is as follows:
3.4 Chapter Summary
3.5 References
Chapter 3 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf