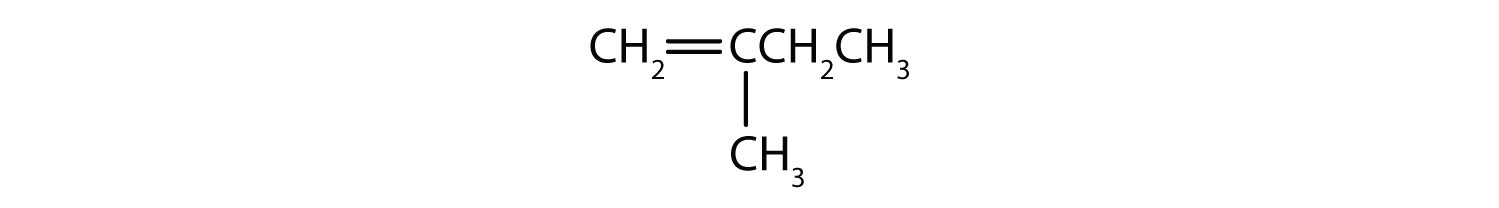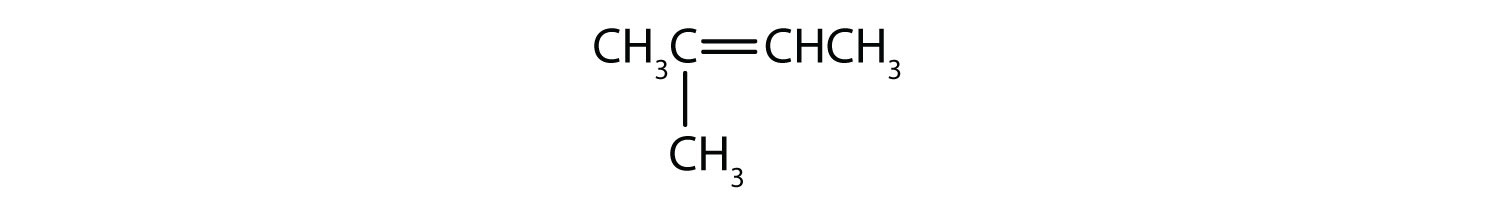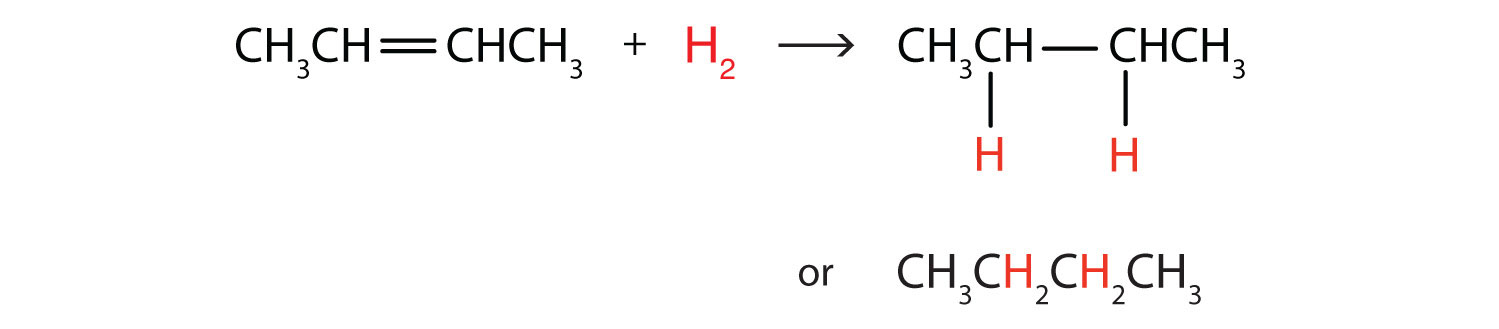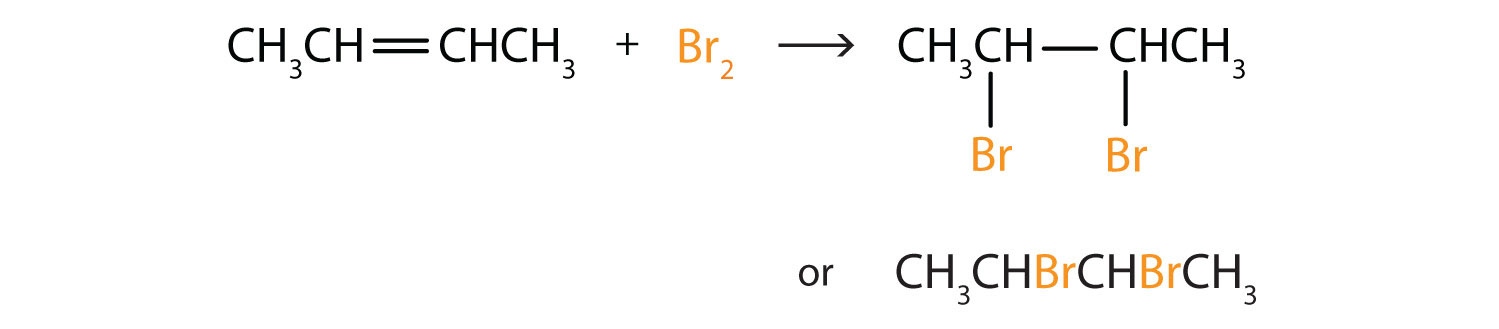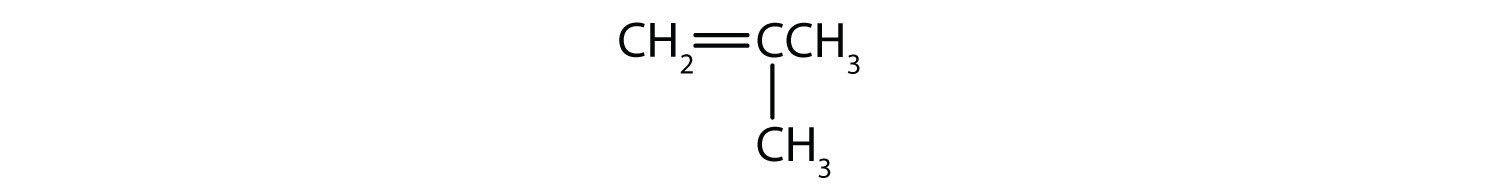Home » Student Resources » Online Chemistry Textbooks » CH105: Consumer Chemistry » CH105: Chapter 8 – Alkenes, Alkynes and Aromatic Compounds
MenuCH105: Consumer Chemistry
Chapter 8 – Alkenes, Alkynes and Aromatic Compounds
This chapter is also available as a downloadable PDF file. Please click here to download: CH105 Chapter 8 PDF file
This text is published under creative commons licensing, for referencing and adaptation, please click here.
Opening Essay
8.1 Alkene and Alkyne Overview
8.2 Properties of Alkenes
Looking Closer: Environmental Note
8.3 Alkynes
8.4 Aromatic Compounds: Benzene
Polycyclic Aromatic Hydrocarbons
8.5 Geometric Isomers
Cis-Trans Nomenclature
E-Z Nomenclature
8.6 Reactions of Alkenes
Addition Reactions
Hydrogenation
Halogenation
Hydrohalogenation
Hydration
Markovnikov’s Rule
Elimination Reactions
Rearrangement Reactions
Substitution Reactions
8.7 Alkene Polymers
The Production of Polyethylene
8.8 Chapter Summary
8.9 References
Opening Essay
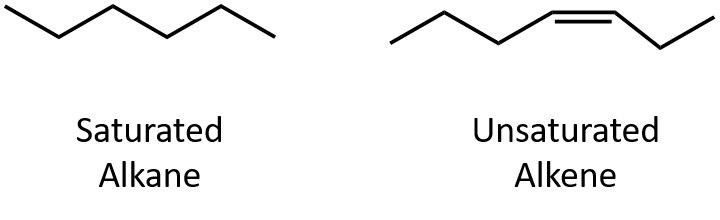
Our modern society is based to a large degree on the chemicals we discuss in this chapter. Most are made from petroleum. In Chapter 7, we noted that alkanes—saturated hydrocarbons—have relatively few important chemical properties other than that they undergo combustion and react with halogens. Unsaturated hydrocarbons—hydrocarbons with double or triple bonds—on the other hand, are quite reactive.
In fact, they serve as building blocks for many familiar plastics—polyethylene, vinyl plastics, acrylics—and other important synthetic materials (e.g., alcohols, antifreeze, and detergents).
Figure 8.1 Common polymers made using alkene building blocks. Upper left, a stainless steel and ultra high molecular weight polyethylene hip replacement. The polyethylene repeating unit is shown in the lower left. Upper middle, shatterproof acrylic plexiglas used to build a large indoor aquarium. The methylacrylate repeating unit is shown in the lower middle. Upper right, common PCV piping used as material being used for sewage and drains. The polyvinylchloride repeating unit is shown in the lower left.
Hip replacement photo provided by: The Science Museum London / Science and Society Picture Library. Plexiglas aquarium photo provided by: Leonard G. PVC pipe installation photo provided by: Steve Tan.
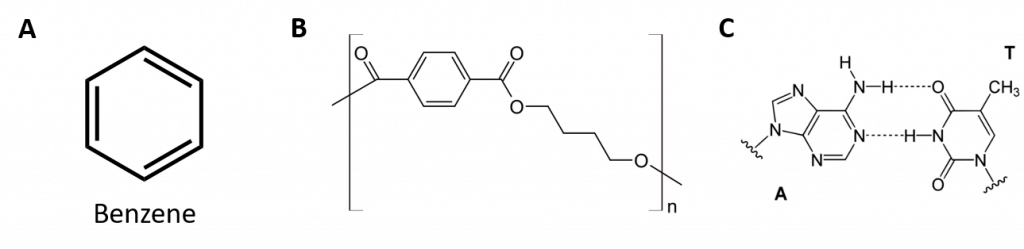
Aromatic hydrocarbons are defined by having 6-membered ring structures with alternating double bonds (Fig 8.2).
Figure 8.2: Aromatic Hydrocarbons. Aromatic hydrocarbons contain the 6-membered benzene ring structure (A) that is characterized by alternating double bonds. Ultradur, PBT is a plastic polymer that contains an aromatic functional group. The repeating monomer of Ultradur is shown in (B). Ultradur can be found in showerheads, toothbrush bristles, plastic housing for fiber-optics cables, and in automobile exterior and interior components. Biologically important molecules, such as deoxyribonucleic acid, DNA (C) also contain an aromatic ring structures.
Thus, they have formulas that can be drawn as cyclic alkenes, making them unsaturated. However, due to the cyclic structure, the properties of aromatic rings are generally quite different, and they do not behave as typical alkenes. Aromatic compounds serve as the basis for many drugs, antiseptics, explosives, solvents, and plastics (e.g., polyesters and polystyrene).
The two simplest unsaturated compounds—ethylene (ethene) and acetylene (ethyne)—were once used as anesthetics and were introduced to the medical field in 1924. However, it was discovered that acetylene forms explosive mixtures with air, so its medical use was abandoned in 1925. Ethylene was thought to be safer, but it too was implicated in numerous lethal fires and explosions during anesthesia. Even so, it remained an important anesthetic into the 1960s, when it was replaced by nonflammable anesthetics such as halothane (CHBrClCF3).
(Back to the Top)
8.1 Alkene and Alkyne Overview
By definition, alkenes are hydrocarbons with one or more carbon–carbon double bonds (R2C=CR2), while alkynes are hydrocarbons with one or more carbon-carbon triple bonds (R–C≡C–R). Collectively, they are called unsaturated hydrocarbons, which are defined as hydrocarbons having one or more multiple (double or triple) bonds between carbon atoms. As a result of the double or triple bond nature, alkenes and alkynes have fewer hydrogen atoms than comparable alkanes with the same number of carbon atoms. Mathematically, this can be indicated by the following general formulas:
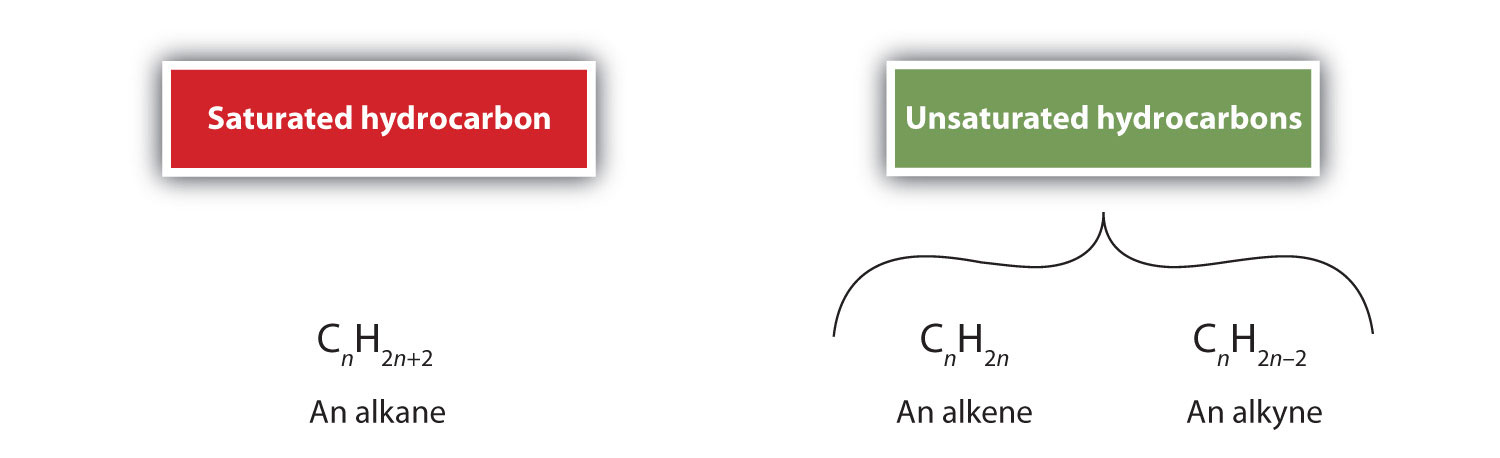
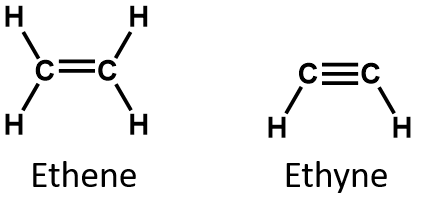
In an alkene, the double bond is shared by the two carbon atoms and does not involve the hydrogen atoms, although the condensed formula does not make this point obvious, ie the condensed formula for ethene is CH2CH2. The double or triple bond nature of a molecule is even more difficult to discern from the molecular formulas. Note that the molecular formula for ethene is C2H4, whereas that for ethyne is C2H2. Thus, until you become more familiar the language of organic chemistry, it is often most useful to draw out line or partially-condensed structures, as shown below:
(Back to the Top)
8.2 Properties of Alkenes
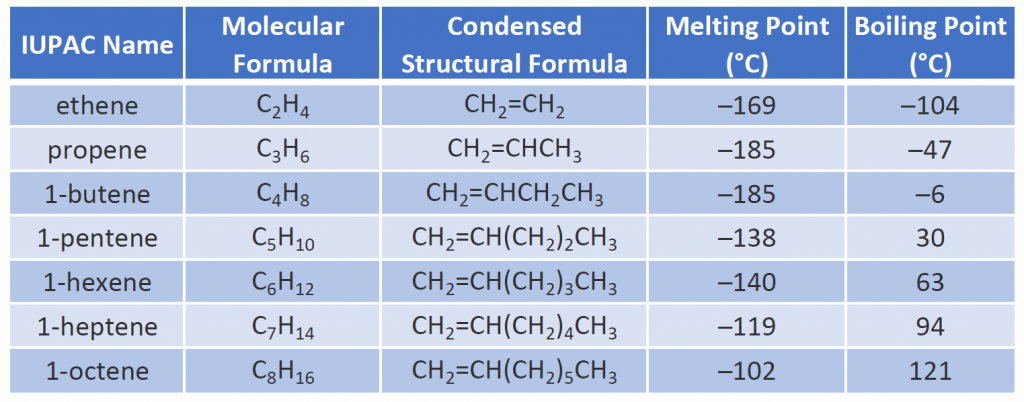
The physical properties of alkenes are similar to those of the alkanes. Table 8.1 shows that the boiling points of straight-chain alkenes increase with increasing molar mass, just as with alkanes. For molecules with the same number of carbon atoms and the same general shape, the boiling points usually differ only slightly, just as we would expect for substances whose molar mass differs by only 2 u (equivalent to two hydrogen atoms). Like other hydrocarbons, the alkenes are insoluble in water but soluble in organic solvents.
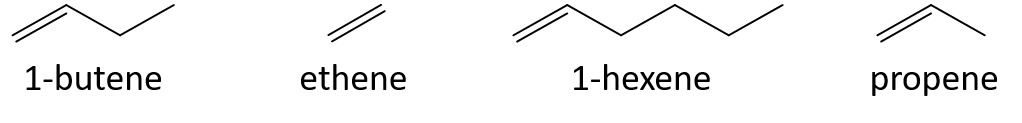
Some representative alkenes—their names, structures, and physical properties—are given in Table 8.1.
Table 8.1 Physical Properties of Some Selected Alkenes
The first two alkenes in Table 8.1 —ethene and propene, are most often called by their common names—ethylene and propylene, respectively. Ethylene is a major commercial chemical. The US chemical industry produces about 25 billion kilograms of ethylene annually, more than any other synthetic organic chemical. More than half of this ethylene goes into the manufacture of polyethylene, one of the most familiar plastics. Propylene is also an important industrial chemical. It is converted to plastics, isopropyl alcohol, and a variety of other products.
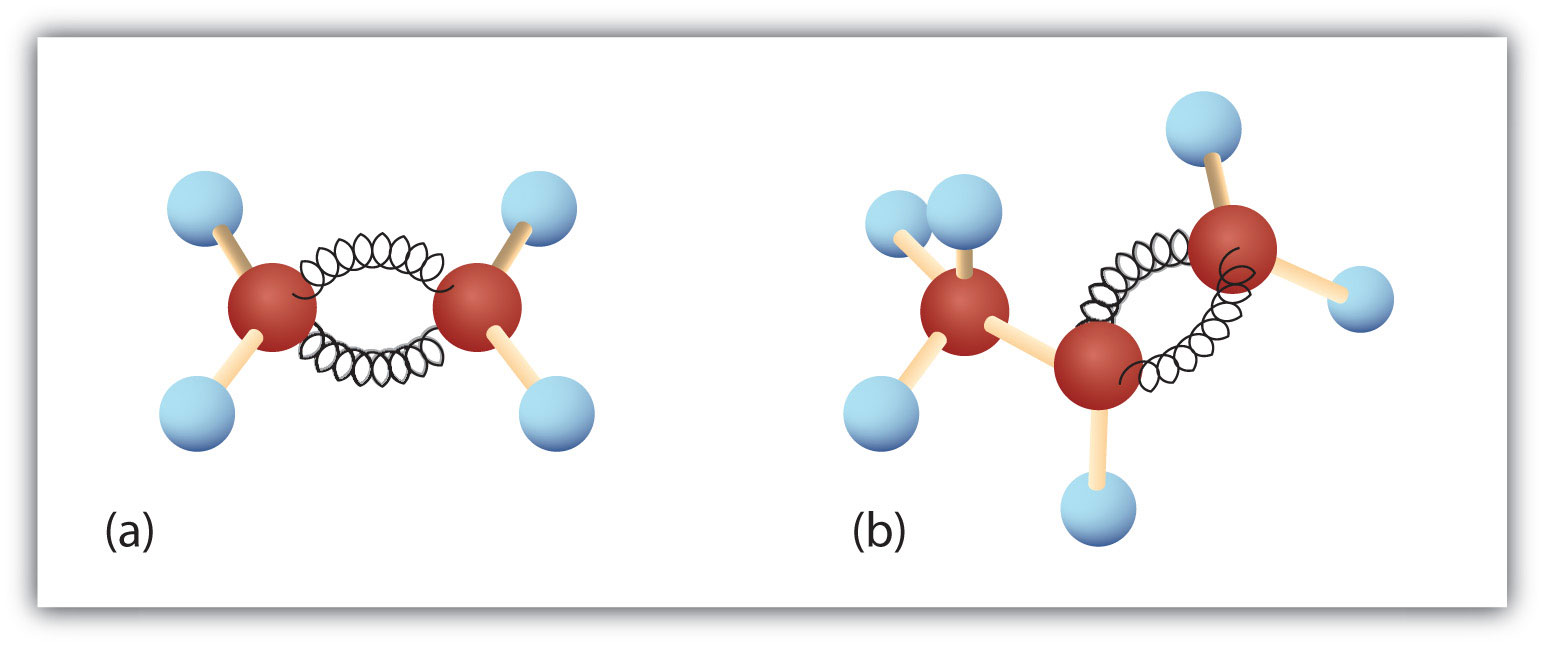
Figure 8.3. Ethene and Propene. The ball-and-spring models of ethene/ethylene (a) and propene/propylene (b) show their respective shapes, especially bond angles.
Looking Closer: Environmental Note
Alkenes occur widely in nature. Ripening fruits and vegetables give off ethylene, which triggers further ripening. Fruit processors artificially introduce ethylene to hasten the ripening process; exposure to as little as 0.1 mg of ethylene for 24 h can ripen 1 kg of tomatoes. Unfortunately, this process does not exactly duplicate the ripening process, and tomatoes picked green and treated this way don’t taste much like vine-ripened tomatoes fresh from the garden.
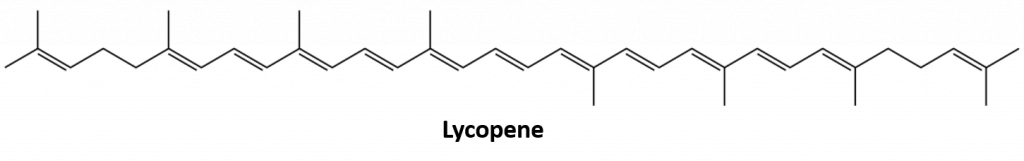
Other alkenes that occur in nature include 1-octene, a constituent of lemon oil, and octadecene (C18H36) found in fish liver. Dienes (two double bonds) and polyenes (three or more double bonds) are also common. Butadiene (CH2=CHCH=CH2) is found in coffee. Lycopene and the carotenes are isomeric polyenes (C40H56) that give the attractive red, orange, and yellow colors to watermelons, tomatoes, carrots, and other fruits and vegetables. Vitamin A, essential to good vision, is derived from a carotene. The world would be a much less colorful place without alkenes.

Figure 8.4 The bright red color of tomatoes is due to lycopene.
Photo from : © Thinkstock; Lycopene structure from: Jeff Dahl
Concept Review Exercises
-
Briefly describe the physical properties of alkenes. How do these properties compare to those of the alkanes?
Answers
-
Alkenes have physical properties (low boiling points, insoluble in water) quite similar to those of their corresponding alkanes.
-
ethene < propene < 1-butene < 1-hexene
Key Takeaway
- The physical properties of alkenes are much like those of the alkanes: their boiling points increase with increasing molar mass, and they are insoluble in water.
Exercises
Answer
-
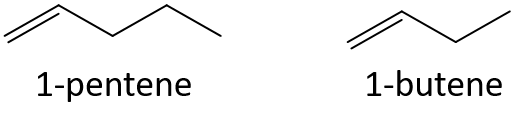
- 1-pentene
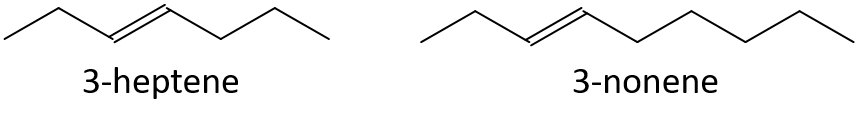
- 3-nonene
Concept Review Exercises
-
Briefly identify the important distinctions between a saturated hydrocarbon and an unsaturated hydrocarbon.
-
Briefly identify the important distinctions between an alkene and an alkane.
-
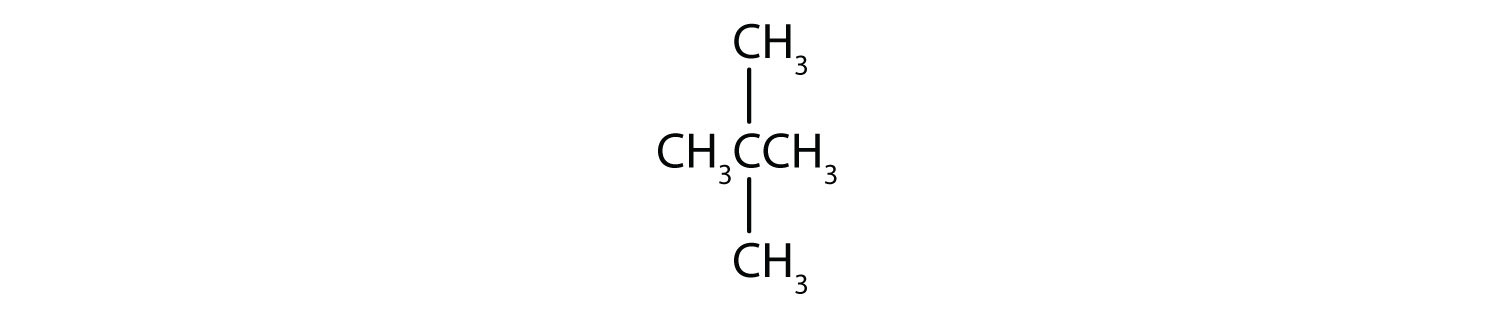
Classify each compound as saturated or unsaturated. Identify each as an alkane, an alkene, or an alkyne.
-

- CH3CH2C≡CCH3
-

-
Answers
-
Unsaturated hydrocarbons have double or triple bonds and are quite reactive; saturated hydrocarbons have only single bonds and are rather unreactive.
-
An alkene has a double bond; an alkane has single bonds only.
-
- saturated; alkane
- unsaturated; alkyne
- unsaturated; alkene
Key Takeaway
- Alkenes are hydrocarbons with a carbon-to-carbon double bond.
8.3 Alkynes
The simplest alkyne—a hydrocarbon with carbon-to-carbon triple bond—has the molecular formula C2H2 and is known by its common name—acetylene (Fig 8.5). Its structure is H–C≡C–H.
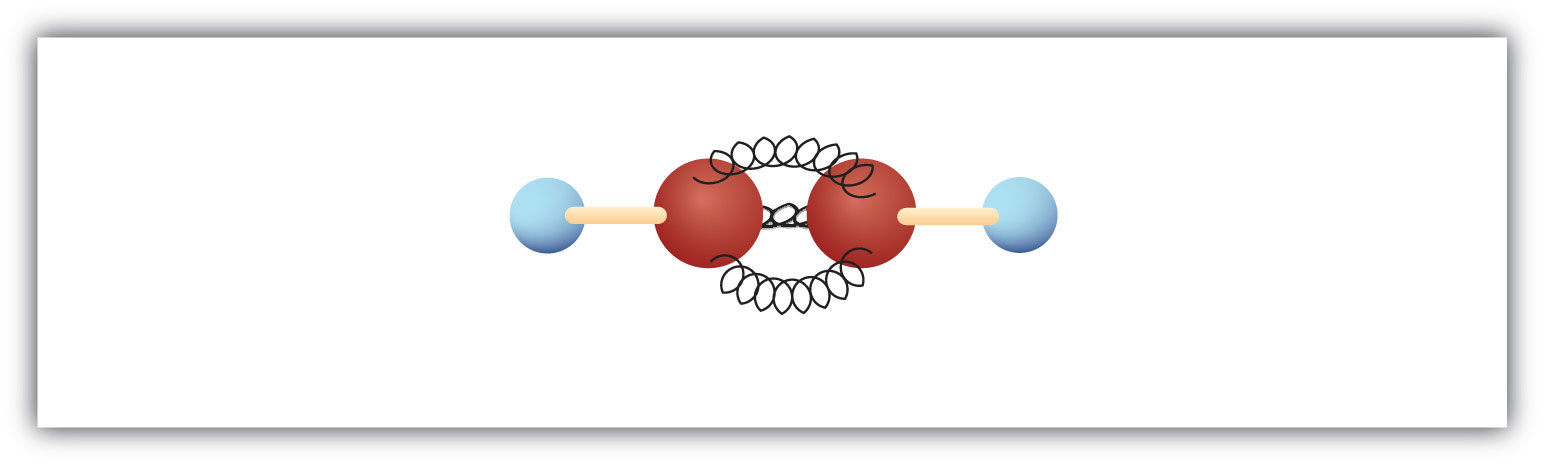
Figure 8.5 Ball-and-Spring Model of Acetylene. Acetylene (ethyne) is the simplest member of the alkyne family.
Note
Acetylene is used in oxyacetylene torches for cutting and welding metals. The flame from such a torch can be very hot. Most acetylene, however, is converted to chemical intermediates that are used to make vinyl and acrylic plastics, fibers, resins, and a variety of other products.
Alkynes are similar to alkenes in both physical and chemical properties. For example, alkynes undergo many of the typical addition reactions of alkenes. The International Union of Pure and Applied Chemistry (IUPAC) names for alkynes parallel those of alkenes, except that the family ending is –yne rather than –ene. The IUPAC name for acetylene is ethyne. The names of other alkynes are illustrated in the following exercises.
Concept Review Exercises
-
Briefly identify the important differences between an alkene and an alkyne. How are they similar?
-
The alkene (CH3)2CHCH2CH=CH2 is named 4-methyl-1-pentene. What is the name of (CH3)2CHCH2C≡CH?
-
Do alkynes show cis-trans isomerism? Explain.
Answers
-
Alkenes have double bonds; alkynes have triple bonds. Both undergo addition reactions.
-
4-methyl-1-pentyne
-
No; a triply bonded carbon atom can form only one other bond. It would have to have two groups attached to show cis-trans isomerism.
Key Takeaway
- Alkynes are hydrocarbons with carbon-to-carbon triple bonds and properties much like those of alkenes.
Exercises
-
Draw the structure for each compound.
- acetylene
- 3-methyl-1-hexyne
-
Draw the structure for each compound.
- 4-methyl-2-hexyne
- 3-octyne
-
Name each alkyne.
- CH3CH2CH2C≡CH
- CH3CH2CH2C≡CCH3
Answers
-
- H–C≡C–H
-

-
- 1-pentyne
- 2-hexyne
(Back to the Top)
8.4 Aromatic Compounds: Benzene
Next we consider a class of hydrocarbons with molecular formulas like those of unsaturated hydrocarbons, but which, unlike the alkenes, do not readily undergo addition reactions. These compounds comprise a distinct class, called aromatic hydrocarbons. Aromatic hydrocarbons are compounds that contain a benzene ring structure.The simplest aromatic compound is benzene (C6H6) and it is of great commercial importance, but it also has noteworthy deleterious health effects (see “To Your Health: Benzene and Us”).
The formula C6H6 seems to indicate that benzene has a high degree of unsaturation. (Hexane, the saturated hydrocarbon with six carbon atoms has the formula C6H14—eight more hydrogen atoms than benzene.) However, despite the seeming low level of saturation, benzene is rather unreactive. This is due to the resonance structure formed from the alternating double bond structure of the aromatic ring.
Note
Benzene is a liquid that smells like gasoline, boils at 80°C, and freezes at 5.5°C. It is the aromatic hydrocarbon produced in the largest volume. It was formerly used to decaffeinate coffee and was a significant component of many consumer products, such as paint strippers, rubber cements, and home dry-cleaning spot removers. It was removed from many product formulations in the 1950s, but others continued to use benzene in products until the 1970s when it was associated with leukemia deaths. Benzene is still important in industry as a precursor in the production of plastics (such as Styrofoam and nylon), drugs, detergents, synthetic rubber, pesticides, and dyes. It is used as a solvent for such things as cleaning and maintaining printing equipment and for adhesives such as those used to attach soles to shoes. Benzene is a natural constituent of petroleum products, but because it is a known carcinogen, its use as an additive in gasoline is now limited.
To Your Health: Benzene and Us
Most of the benzene used commercially comes from petroleum. It is employed as a starting material for the production of detergents, drugs, dyes, insecticides, and plastics. Once widely used as an organic solvent, benzene is now known to have both short- and long-term toxic effects. The inhalation of large concentrations can cause nausea and even death due to respiratory or heart failure, while repeated exposure leads to a progressive disease in which the ability of the bone marrow to make new blood cells is eventually destroyed. This results in a condition called aplastic anemia, in which there is a decrease in the numbers of both the red and white blood cells.
Concept Review Exercises
-
How do the typical reactions of benzene differ from those of the alkenes?
-
Briefly describe the bonding in benzene.
-
What does the circle mean in the chemist’s representation of benzene?
Answers
-
Benzene is rather unreactive toward addition reactions compared to an alkene.
-
Valence electrons are shared equally by all six carbon atoms (that is, the electrons are delocalized).
-
The six electrons are shared equally by all six carbon atoms.
Recognizing Aromatic Compounds
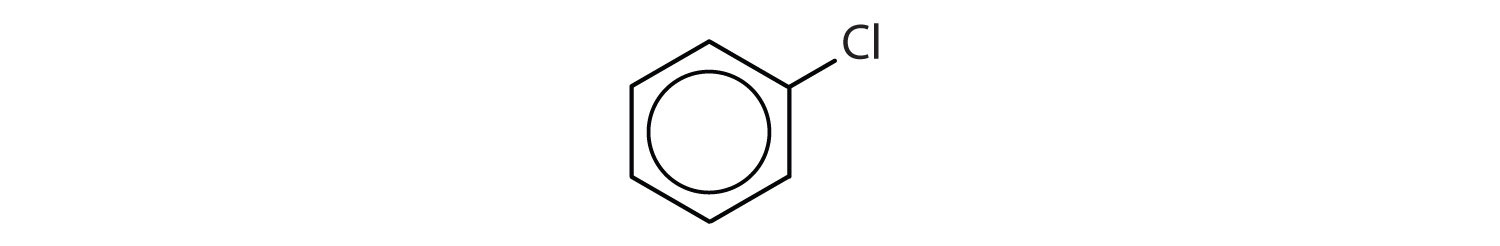
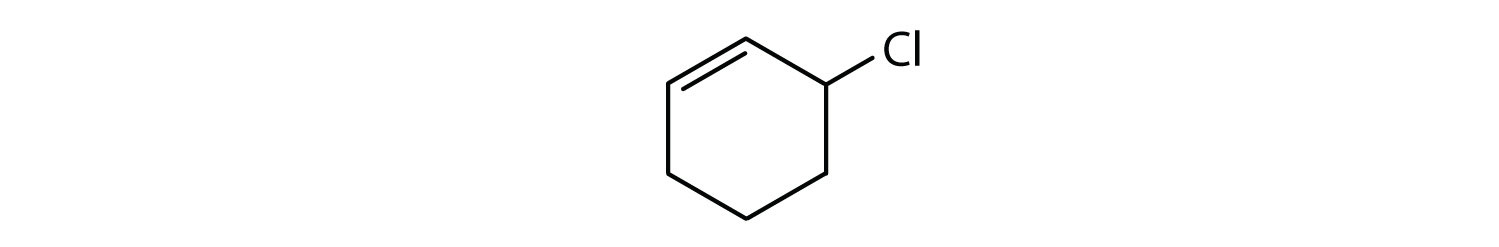
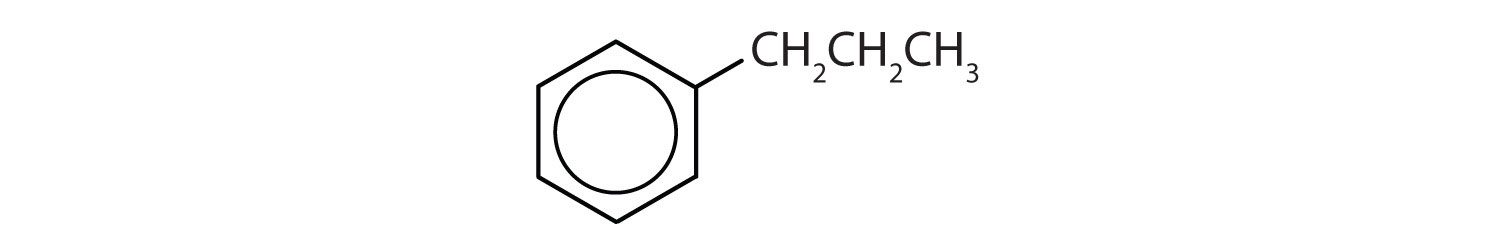
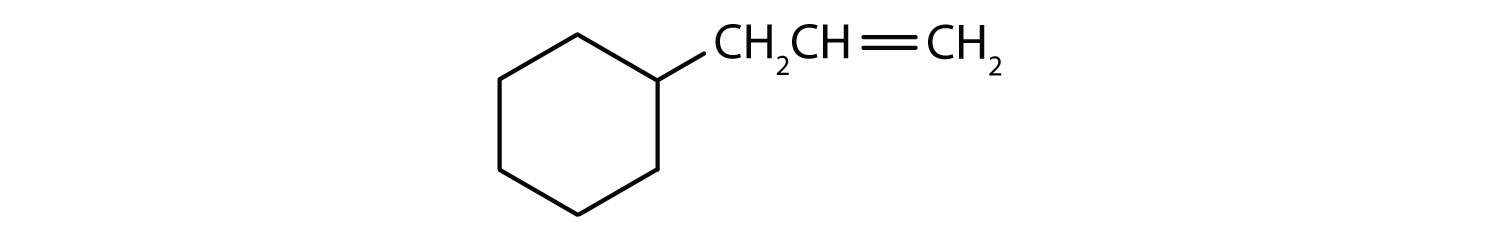
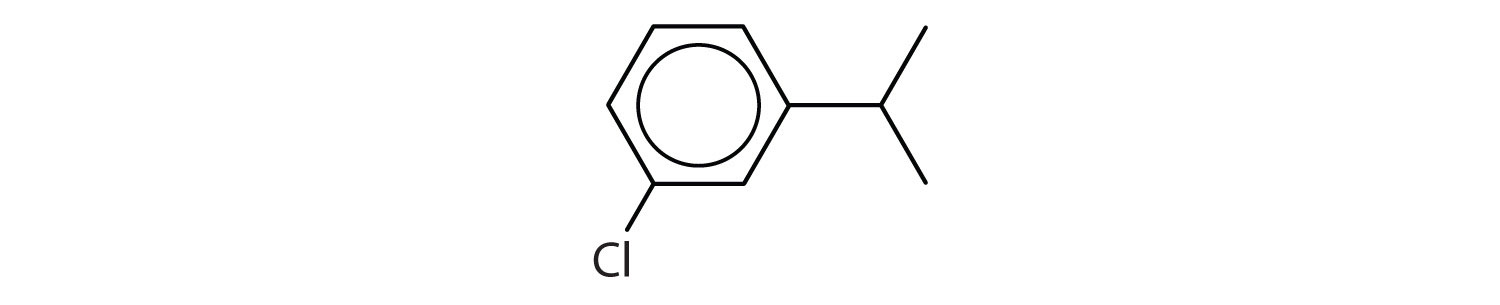
Which compounds are aromatic?
Solution
- The compound has a benzene ring (with a chlorine atom substituted for one of the hydrogen atoms); it is aromatic.
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
- The compound has a benzene ring (with a propyl group substituted for one of the hydrogen atoms); it is aromatic.
- The compound is cyclic, but it does not have a benzene ring; it is not aromatic.
Skill-Building Exercise
Which compounds are aromatic?
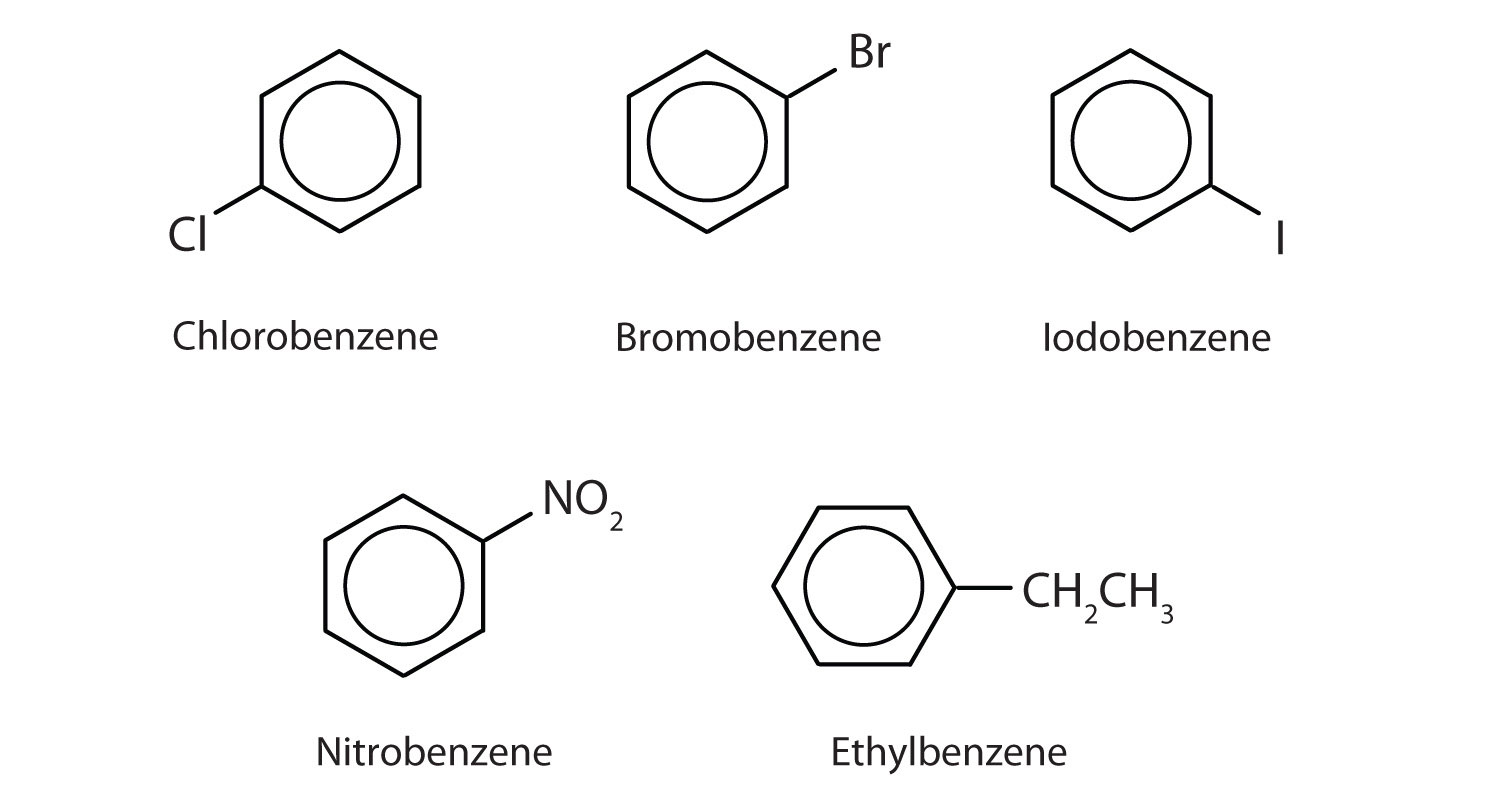
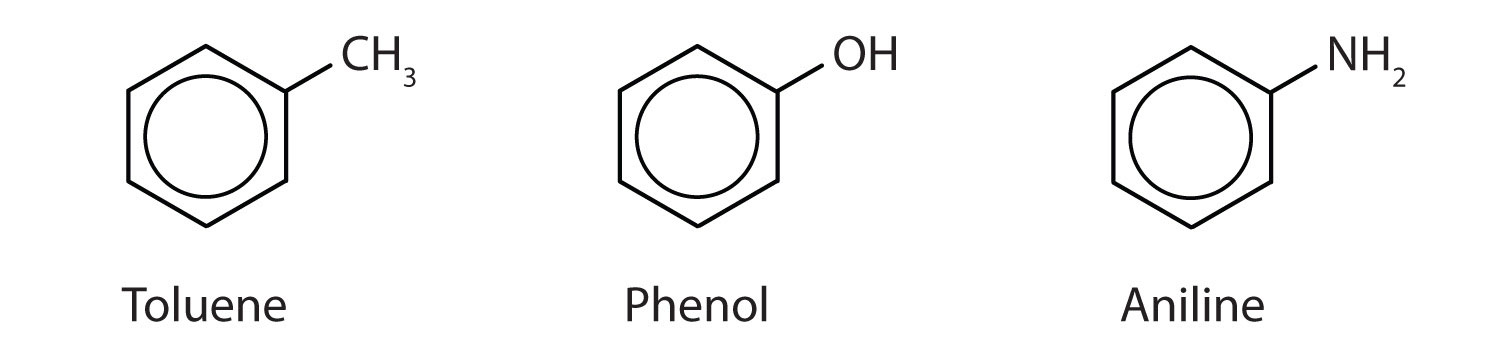
In the International Union of Pure and Applied Chemistry (IUPAC) system, aromatic hydrocarbons are named as derivatives of benzene. Five examples are shown below. In these structures, it is immaterial whether the single substituent is written at the top, side, or bottom of the ring: a hexagon is symmetrical, and therefore all positions are equivalent.

These compounds are named in the usual way with the group that replaces a hydrogen atom named as a substituent group: Cl as chloro, Br as bromo, I as iodo, NO2 as nitro, and CH3CH2 as ethyl.
Although some compounds are referred to exclusively by IUPAC names, some are more frequently denoted by common names, as is indicated below.

-
Key Takeaway
- Aromatic hydrocarbons appear to be unsaturated, but they have a special type of bonding and do not undergo addition reactions.
(Back to the Top)
Polycyclic Aromatic Hydrocarbons
Some common aromatic hydrocarbons consist of fused benzene rings—rings that share a common side. These compounds are called polycyclic aromatic hydrocarbons (PAHs)An aromatic hydrocarbon consisting of fused benzene rings sharing a common side..
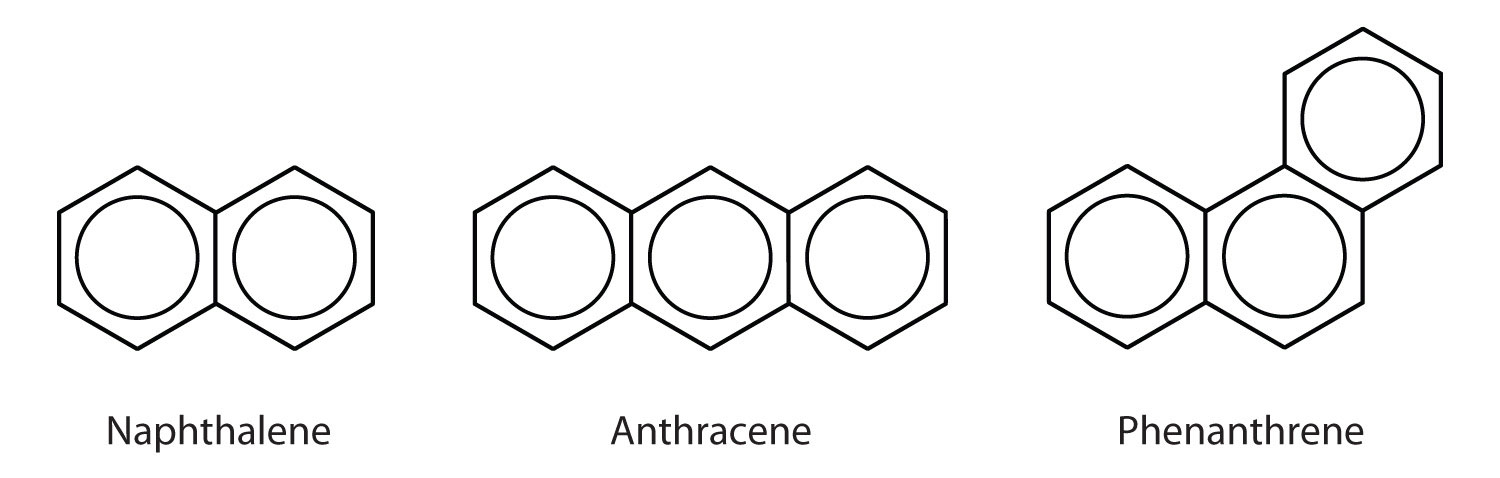
The three examples shown here are colorless, crystalline solids generally obtained from coal tar. Naphthalene has a pungent odor and is used in mothballs. Anthracene is used in the manufacture of certain dyes. Steroids, including cholesterol and the hormones, estrogen and testosterone, contain the phenanthrene structure.
To Your Health: Polycyclic Aromatic Hydrocarbons and Cancer
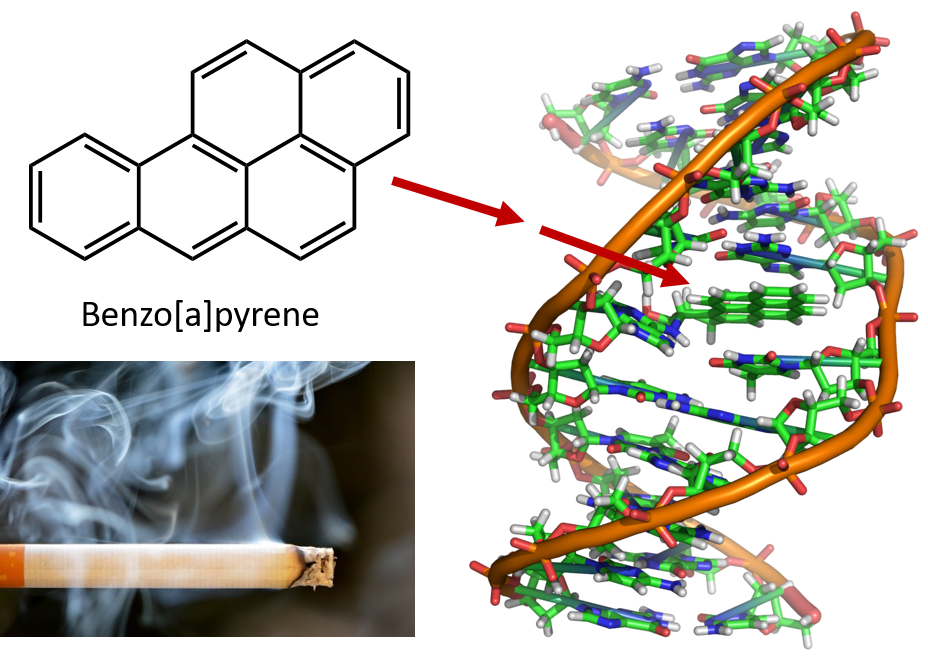
The intense heating required for distilling coal tar results in the formation of PAHs. For many years, it has been known that workers in coal-tar refineries are susceptible to a type of skin cancer known as tar cancer. Investigations have shown that a number of PAHs are carcinogens. One of the most active carcinogenic compounds, benzopyrene, occurs in coal tar and has also been isolated from cigarette smoke, marijuana smoke, automobile exhaust gases, and charcoal-broiled steaks. It is estimated that more than 1,000 t of benzopyrene are emitted into the air over the United States each year. Only a few milligrams of benzopyrene per kilogram of body weight are required to induce cancer in experimental animals.
Figure 8.6 Benzo[a]pyrene is a polycyclic aromatic hydrocarbon produced as a byproduct in coal tar, cigarette and marijuana smoke, and in charbroiled steaks. Benzo[a]pyrene is metabolized to produce biologically active compounds that can form physical adducts on DNA molecules. These adducts can cause genetic mutations that cause cancer.
Photo of cigarette smoke
Biologically Important Compounds with Benzene Rings
Substances containing the benzene ring are common in both animals and plants, although they are more abundant in the latter. Plants can synthesize the benzene ring from carbon dioxide, water, and inorganic materials. Animals cannot synthesize it, but they are dependent on certain aromatic compounds for survival and therefore must obtain them from food. Phenylalanine, tyrosine, and tryptophan (essential amino acids) and vitamins K, B2 (riboflavin), and B9 (folic acid) all contain the benzene ring. Many important drugs, a few of which are shown in Table 8.2 also feature a benzene ring.
Note
So far we have studied only aromatic compounds with carbon-containing rings. However, many cyclic compounds have an element other than carbon atoms in the ring. Organic ring structures that contain an atom other than carbon are called heterocyclic compounds., Heterocyclic aromatic compounds also have unique and medically relevant properties.
Table 8.2 Some Drugs That Contain a Benzene Ring
| Name | Structure |
|---|---|
| aspirin |
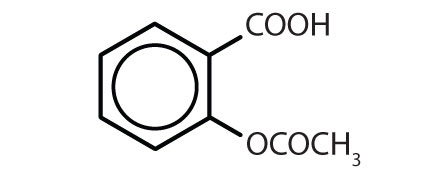 |
| acetaminophen |
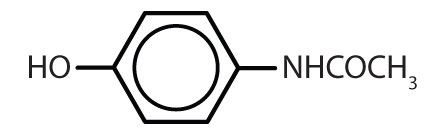 |
| ibuprofen |
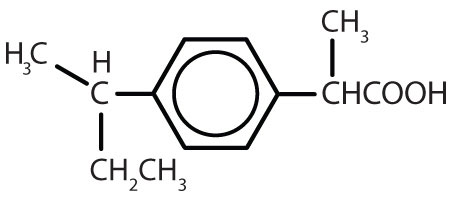 |
| amphetamine |
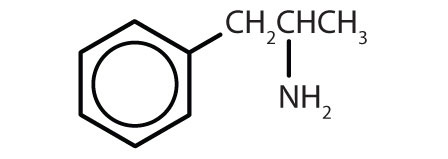 |
| sulfanilamide |
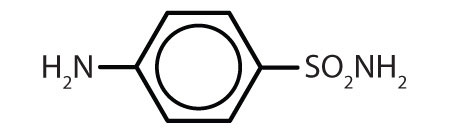 |
8.5 Geometric Isomers
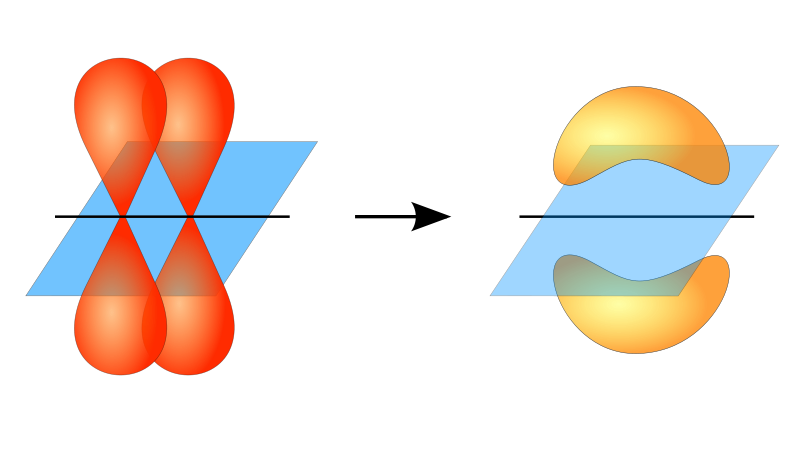
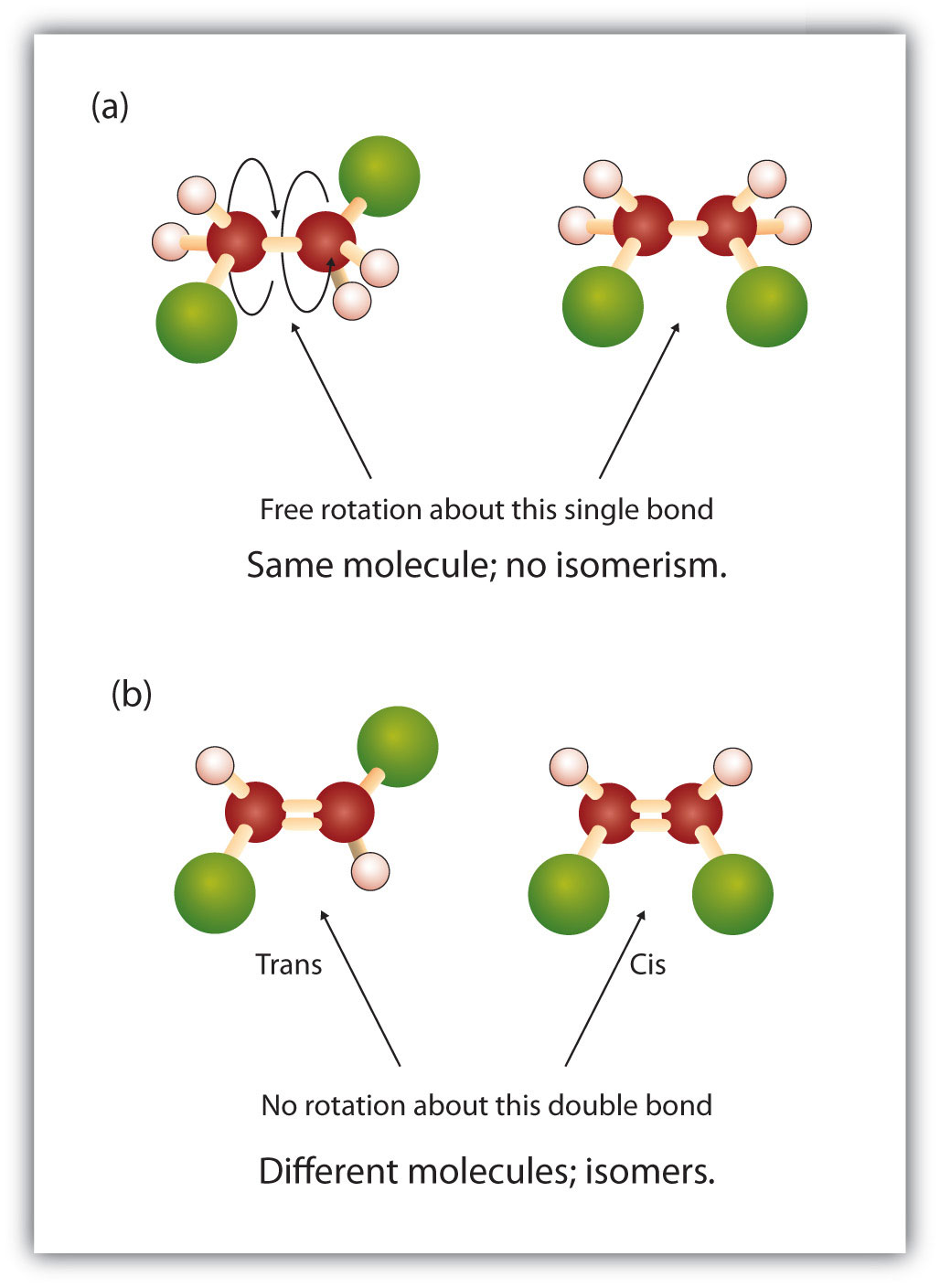
Within alkane structure there is free rotation about the carbon-to-carbon single bonds (C–C). In contrast, the structure of alkenes requires that the carbon atoms form a double bond. Double bonds between elements are created using p-orbital shells (also called pi orbitals). These orbital shells are shaped like dumbbells rather than the circular orbitals used in single bonds. This prevents the free rotation of the carbon atoms around the double bond, as it would cause the double bond to break during the rotation (Figure 8.7). Thus, a single bond is analogous to two boards nailed together with one nail. The boards are free to spin around the single nail. A double bond, on the other hand, is analogous to two boards nailed together with two nails. In the first case you can twist the boards, while in the second case you cannot twist them.
Figure 8.7 The formation of double bonds requires the use of pi-bonds. For molecules to create double bonds, electrons must share overlapping pi-orbitals between the two atoms. This requires the dumbbell-shaped pi-orbitals (show on the left) to remain in a fixed conformation during the double bond formation. This allows for the formation of electron orbitals that can be shared by both atoms (shown on the right). Rotation around the double bond would cause the pi orbitals to be misaligned, breaking the double bond.
Diagram provided from: JoJanderivative work – Vladsinger (talk)
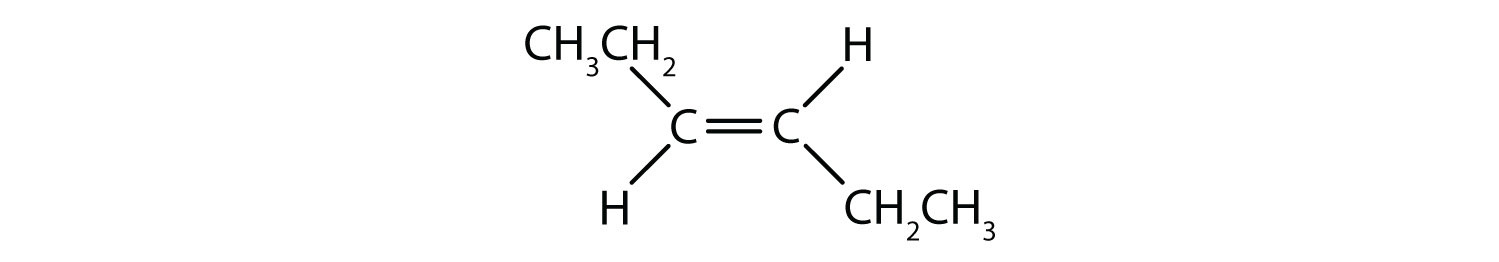
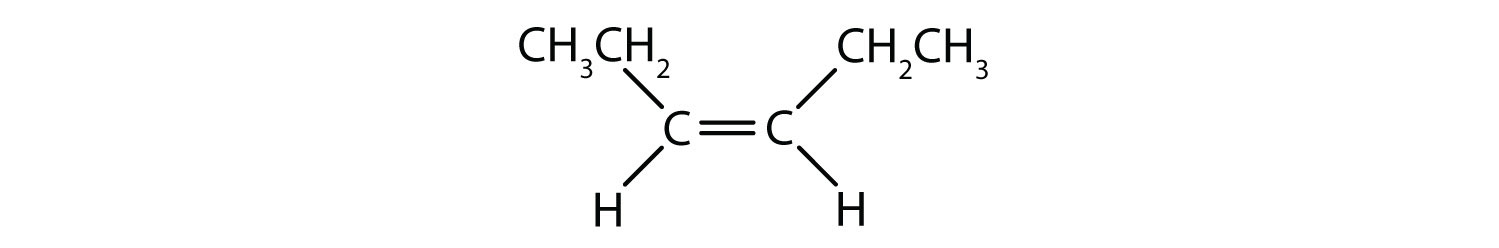
The fixed and rigid nature of the double bond creates the possibility of an additional chiral center, and thus, the potential for stereoisomers. New stereoisomers form if each of the carbons involved in the double bond has two different atoms or groups attached to it. For example, look at the two chlorinated hydrocarbons in Figure 8.8. In the upper figure, the halogenated alkane is shown. Rotation around this carbon-carbon bond is possible and does not result in different isomer conformations. In the lower diagram, the halogenated alkene has restricted rotation around the double bond. Note also that each carbon involved in the double bond is also attached to two different atoms (a hydrogen and a chlorine). Thus, this molecules can form two stereoisomers: one that has the two chlorine atoms on the same side of the double bond, and the other where the chlorines reside on opposite sides of the double bond.
Figure 8.8 Alkene Double Bonds Can Form Geometric Isomers. (a) Shows the free rotation around a carbon-carbon single bond in the alkane structure. (b) Shows the fixed position of the carbon-carbon double bond that leads to geometic (spatial) isomers.
Click Here for a Kahn Academy Video Tutorial on Alkene Structure.
For this section, we are not concerned with the naming that is also included in this video tutorial.(Note: All Khan Academy content is available for free using CC-BY-NC-SA licensing at www.khanacademy.org )
Cis-Trans Nomenclature
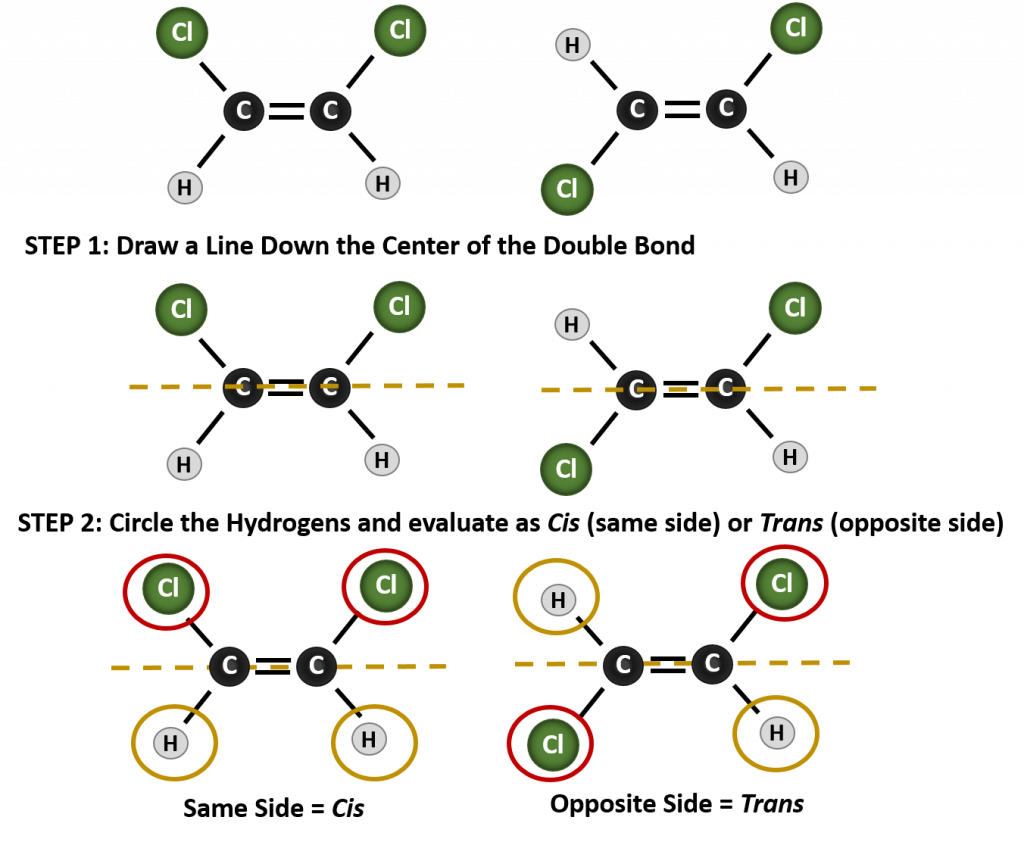
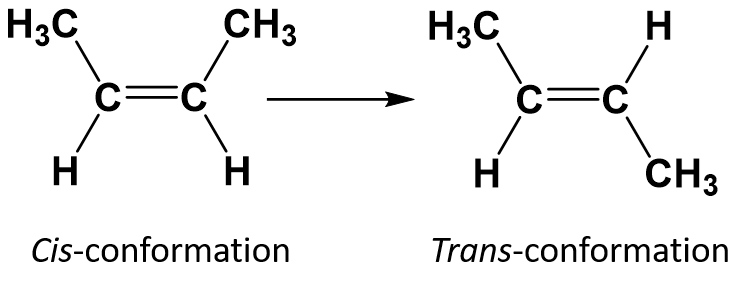
The cis-trans naming system can be used to distinguish simple isomers, where each carbon of the double bond has a set of identical groups attached to it. For example, in Figure 8.8b, each carbon involved in the double bond, has a chlorine attached to it, and also has hydrogen attached to it. The cis and trans system, identifies whether identical groups are on the same side (cis) of the double bond or if they are on the opposite side (trans) of the double bond. For example, if the hydrogen atoms are on the opposite side of the double bond, the bond is said to be in the trans conformation. When the hydrogen groups are on the same side of the double bond, the bond is said to be in the cis conformation. Notice that you could also say that if both of the chlorine groups are on the opposite side of the double bond, that the molecule is in the trans conformation or if they are on the same side of the double bond, that the molecule is in the cis conformation.
To determine whether a molecule is cis or trans, it is helpful to draw a dashed line down the center of the double bond and then circle the identical groups, as shown in figure 8.9. Both of the molecules shown in Figure 8.9, are named 1,2-dichloroethene. Thus, the cis and trans designation, only defines the stereochemistry around the double bond, it does not change the overall identity of the molecule. However, cis and trans isomers often have different physical and chemical properties, due to the fixed nature of the bonds in space.
Figure 8.9 A Guide for Determining Cis or Trans Conformations.
Click Here for a Kahn Academy Video Tutorial on Cis/Trans Isomerization
(Note: All Khan Academy content is available for free using CC-BY-NC-SA licensing at www.khanacademy.org )
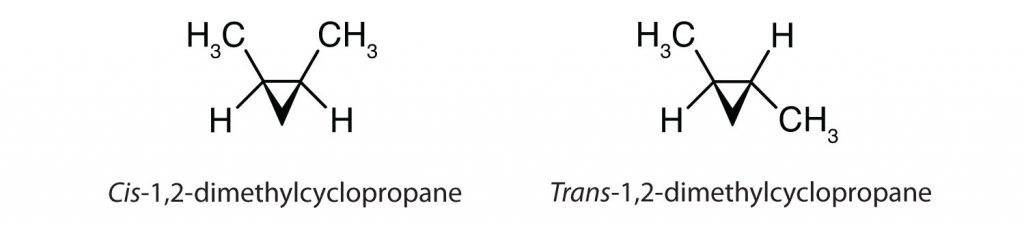
Cis-trans isomerism also occurs in cyclic compounds. In ring structures, groups are unable to rotate about any of the ring carbon–carbon bonds. Therefore, groups can be either on the same side of the ring (cis) or on opposite sides of the ring (trans). For our purposes here, we represent all cycloalkanes as planar structures, and we indicate the positions of the groups, either above or below the plane of the ring.
To Your Health
Possibly the most common place that you will hear reference to cis-trans conformations in everyday life is at the supermarket or your doctor’s office. It relates to our consumption of dietary fats. Inappropriate or excessive consumption of dietary fats has been linked to many health disorders, such as diabetes and atherosclerosis, and coronary heart disease. So what are the differences between saturated and unsaturated fats and what are trans fats and why are they such a health concern?
Figure 8.10 Common Sources of Dietary Fats.
Photo from: TyMaHe
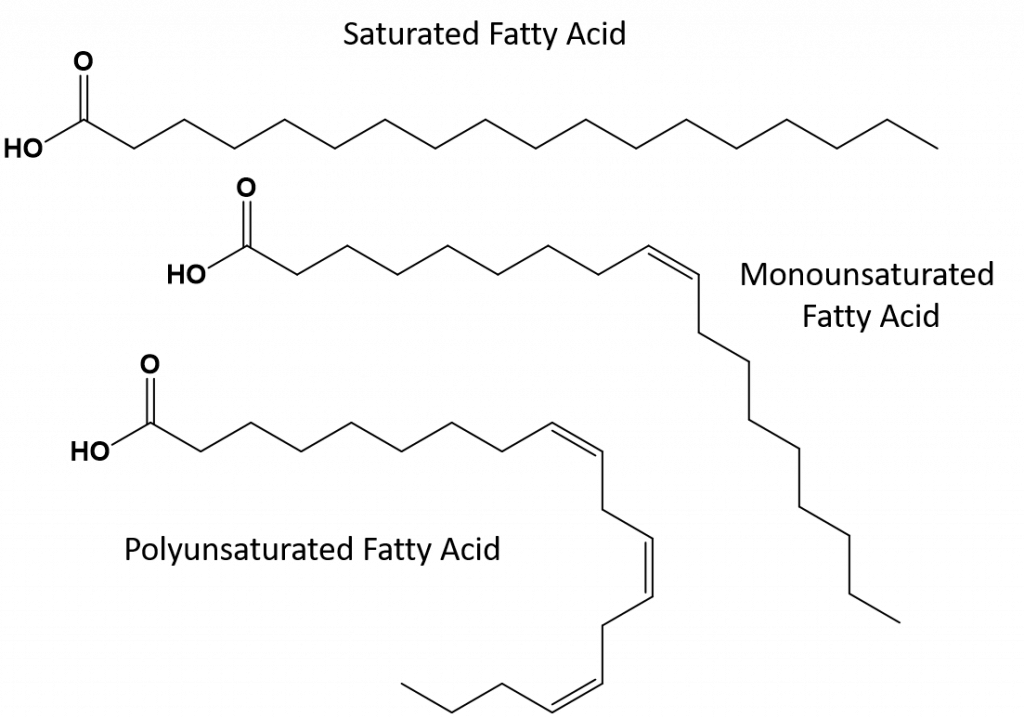
The most common form of dietary fats and the main constituent of body fat in humans and other animals are the triglycerides (TAGs). TAGs, as shown in figure 8.10, are built from one molecule of glycerol and three molecules of fatty acids that are linked together by an ester bond. In this section, we will focus on the structure of the long fatty acid tails, which can be composed of alkane or alkene structures. Chapter 10 will focus more on the formation of the ester bonds.
Figure 8.11. Example of a Triglyceride (TAG) Structure. Notice that each triglyceride has three long chain fatty acids extending from the glycerol backbone. Each fatty acid can have different degrees of saturation and unsaturation.
Structure adapted from: Wolfgang Schaefer
Fats that are fully saturated will only have fatty acids with long chain alkane tails. They are said to be ‘saturated‘ with hydrogen atoms. Saturated fats are common in the American diet and are found in red meat, dairy products like milk, cheese and butter, coconut oil, and are found in many baked goods. Saturated fats are typically solids at room temperature. This is because the long chain alkanes can stack together having more intermolecular London dispersion forces. This gives saturated fats higher melting points and boiling points than the unsaturated fats found in many vegetable oils.
Most of the unsaturated fats found in nature are in the cis-conformation, as shown in Figure 8.11. Note that the fatty acids shown in Figure 8.11 are drawn for convenience, so that they are easy to look at and do not take up too much space on the paper, but the bond angles written do not adequately portray the true spatial orientation of the molecules. When the fatty acids from the TAG shown in Figure 8.11 are drawn with correct bond angles, it is easy to see that cis-double bonds cause bends in the alkene chain (Fig. 8.12).
Figure 8.12 Cis-Double Bonds Cause Bends in Fatty Acid Structure
Thus, monounsaturated and polyunsaturated fats cannot stack together as easily and do not have as many intermolecular attractive forces when compared with saturated fats. As a result, they have lower melting points and boiling points and tend to be liquids at room temperature. It has been shown that the reduction or replacement of saturated fats with mono- and polyunsaturated fats in the diet, helps to reduce levels of the low-density-lipoprotein (LDL) form of cholesterol, which is a risk factor for coronary heart disease.
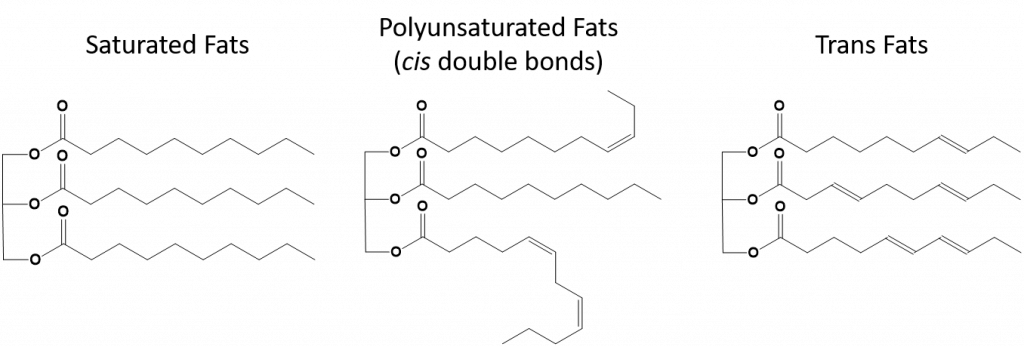
Trans-fats, on the other hand, contain double bonds that are in the trans conformation. Thus, the shape of the fatty acids is linear, similar to saturated fats. Trans fats also have similar melting and boiling points when compared with saturated fats. However, unlike saturated fats, trans-fats are not commonly found in nature and have negative health impacts. Trans-fats occur mainly as a by-product in food processing (mainly the hydrogenation process to create margarines and shortening) or during cooking, especially deep fat frying. In fact, many fast food establishments use trans fats in their deep fat frying process, as trans fats can be used many times before needing to be replaced. Consumption of trans fats raise LDL cholesterol levels in the body (the bad cholesterol that is associated with coronary heart disease) and tend to lower high density lipoprotein (HDL) cholesterol (the good cholesterol within the body). Trans fat consumption increases the risk for heart disease and stroke, and for the development of type II diabetes. The risk has been so highly correlated that many countries have banned the use of trans fats, including Norway, Sweden, Austria and Switzerland. Within the United States, the Food and Drug Administration (FDA) has recently passed a measure to phase out the use of trans fats in foods by 2018. This measure is estimated to prevent 20,000 heart attacks and 7,000 deaths per year.
Figure 8.13 Structural differences in saturated, polyunsaturated and trans fats.
Click Here for a Kahn Academy Video Tutorial on Saturated-, Unsaturated-, and Trans-Fats
(Note: All Khan Academy content is available for free using CC-BY-NC-SA licensing at www.khanacademy.org )
Key Factors for Determining Cis/Trans Isomerization
- The compound needs to contain a double or triple bond, or have a ring structure that will not allow free rotation around the carbon-carbon bond.
- The compound needs to have two non-identical groups attached to each carbon involved in the carbon-carbon double or triple bond.
Worked Example
Which compounds can exist as cis-trans (geometric) isomers? Draw them.
- CHCl=CHBr
- CH2=CBrCH3
- (CH3)2C=CHCH2CH3
- CH3CH=CHCH2CH3
Solution
All four structures have a double bond and thus meet rule 1 for cis-trans isomerism.
-
This compound meets rule 2; it has two nonidentical groups on each carbon atom (H and Cl on one and H and Br on the other). It exists as both cis and trans isomers:

- This compound has two hydrogen atoms on one of its doubly bonded carbon atoms; it fails rule 2 and does not exist as cis and trans isomers.

- This compound has two methyl (CH3) groups on one of its doubly bonded carbon atoms. It fails rule 2 and does not exist as cis and trans isomers.

-
This compound meets rule 2; it has two nonidentical groups on each carbon atom and exists as both cis and trans isomers:

Skill-Building Exercise
-
Which compounds can exist as cis-trans isomers? Draw them.
- CH2=CHCH2CH2CH3
- CH3CH=CHCH2CH3
- CH3CH2CH=CHCH2CH3
-

-

Concept Review Exercises
-
What are cis-trans (geometric) isomers? What two types of compounds can exhibit cis-trans isomerism?
-
Classify each compound as a cis isomer, a trans isomer, or neither.
-
Answers
-
Cis-trans isomers are compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Alkenes and cyclic compounds can exhibit cis-trans isomerism.
-
- trans
- cis
- cis
- neither
Key Takeaway
- Cis-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two different groups on each carbon atom involved in the chemical bond.
(Back to the Top)
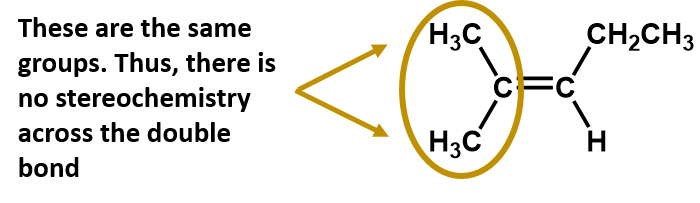
E-Z Nomenclature
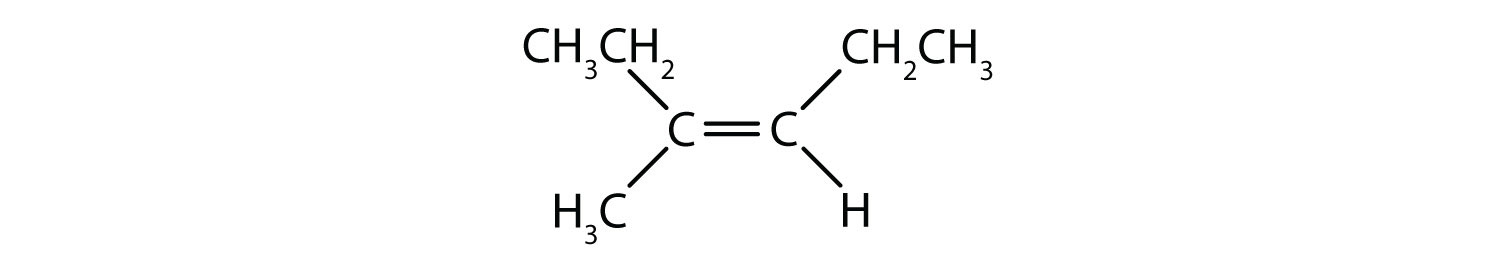
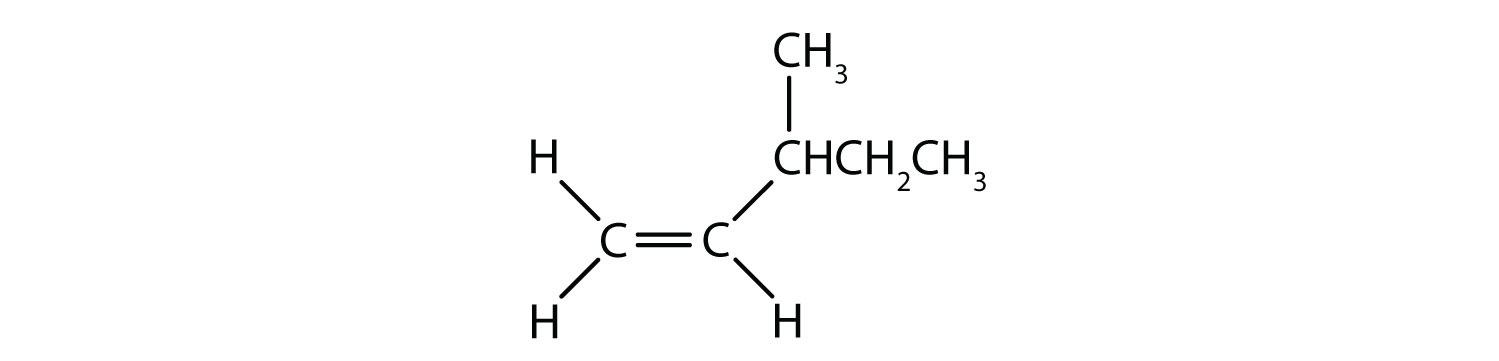
The situation becomes more complex when there are 4 different groups attached to the carbon atoms involved in the formation of the double bond. The cis-trans naming system cannot be used in this case, because there is no reference to which groups are being described by the nomenclature. For example, in the molecule below, you could say that the chlorine is trans to the bromine group, or you could say the chlorine is cis to the methyl (CH3) group. Thus, simply writing cis or trans in this case does not clearly delineate the spatial orientation of the groups in relation to the double bond.
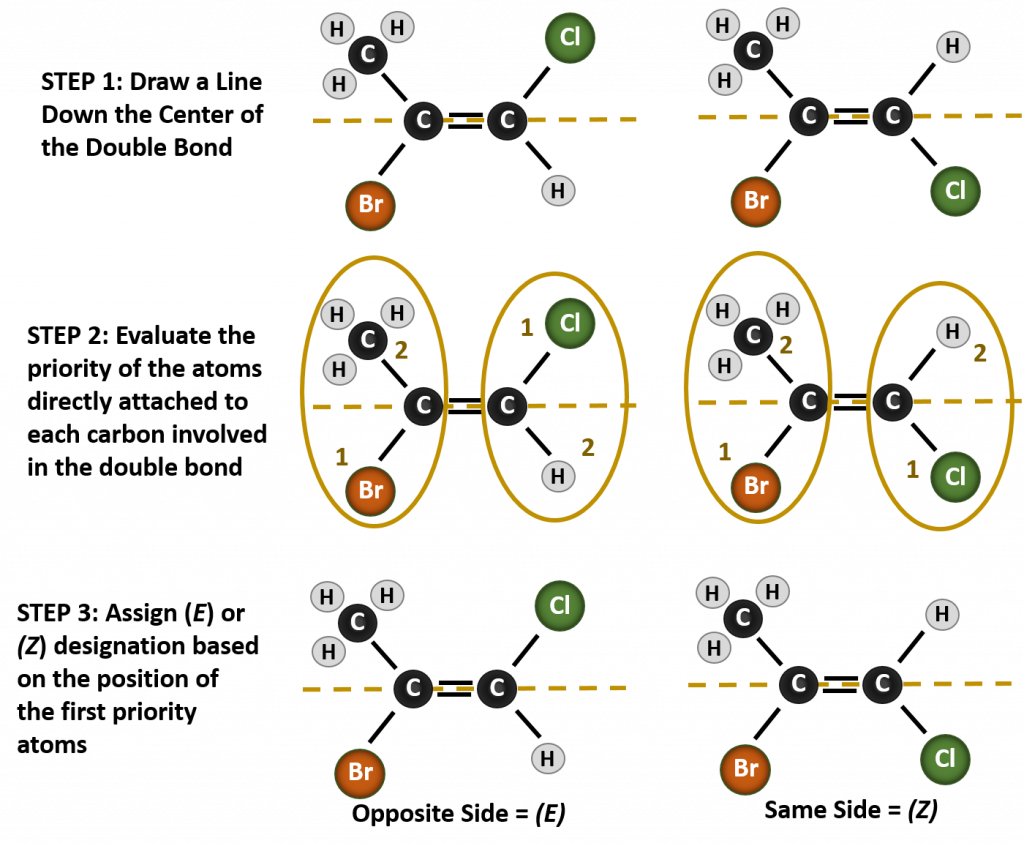
Naming the different stereoisomers formed in this situation, requires knowledge of the priority rules. Recall from chapter 5 that in the Cahn-Ingold-Prelog (CIP) priority system, the groups that are attached to the chiral carbon are given priority based on their atomic number (Z). Atoms with higher atomic number (more protons) are given higher priority (i.e. S > P > O > N > C > H). For this nomenclature system the designations of (Z) and (E) are used instead of the cis/trans system. (E) comes from the German word entgegen, or opposite. Thus, when the higher priority groups are on the opposite side of the double bond, the bond is said to be in the (E) conformation. (Z), on the other hand, comes from the German word zusammen, or together. Thus, when the higher priority groups are on the same side of the double bond, the bond is said to be in the (Z) conformation. Figure 8.14 shows the steps used in assigning the (E) or (Z) conformations of a molecule.
Figure 8.14 Steps used to assign the (E) and (Z) Conformations.
Click Here for a Kahn Academy Video Tutorial on E/Z Isomerization.
(Note: All Khan Academy content is available for free using CC-BY-NC-SA licensing at www.khanacademy.org )
(Back to the Top)
8.6 Reactions of Alkenes
As we saw in Chapter 7, small alkanes can be formed by the process of thermal cracking. This process also produces alkenes and alkynes. In comparison to alkanes, alkenes and alkynes are much more reactive. In fact, alkenes serve as the starting point for the synthesis of many drugs, explosives, paints, plastics and pesticides. Alkanes can undergo five major types of reactions: (1) Combustion Reactions, (2) Addition Reactions, (3) Elimination Reactions, (4) Substitution Reactions, and (5) Rearrangement Reactions. Since combustion reactions were covered heavily in Chapter 7, and combustion reactions with alkenes are not significantly different than combustion reactions with alkanes, this section will focus on the later four reaction types.
Addition Reactions
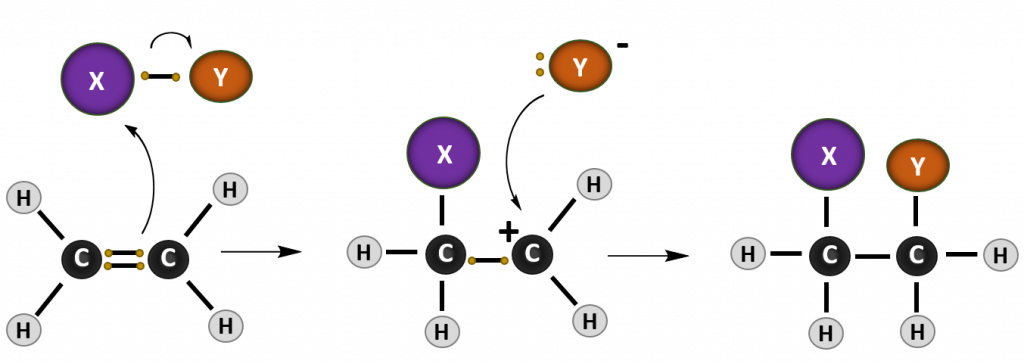
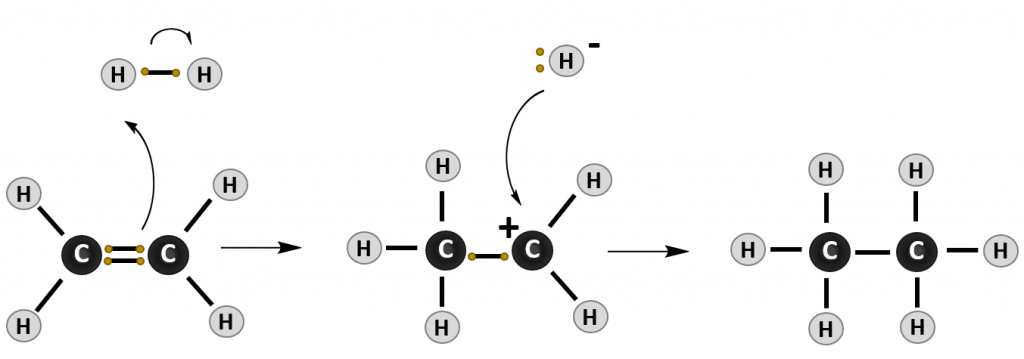
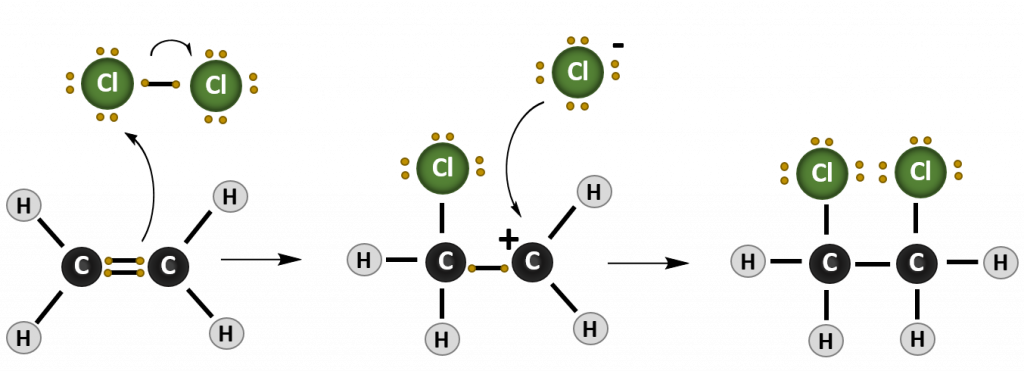
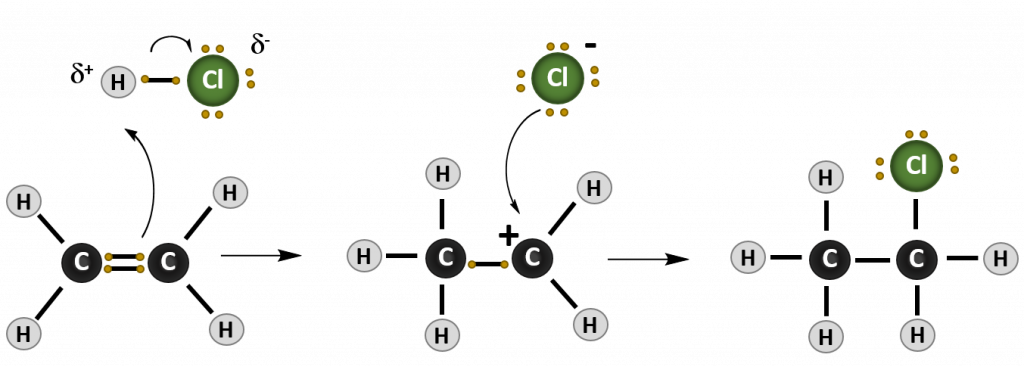
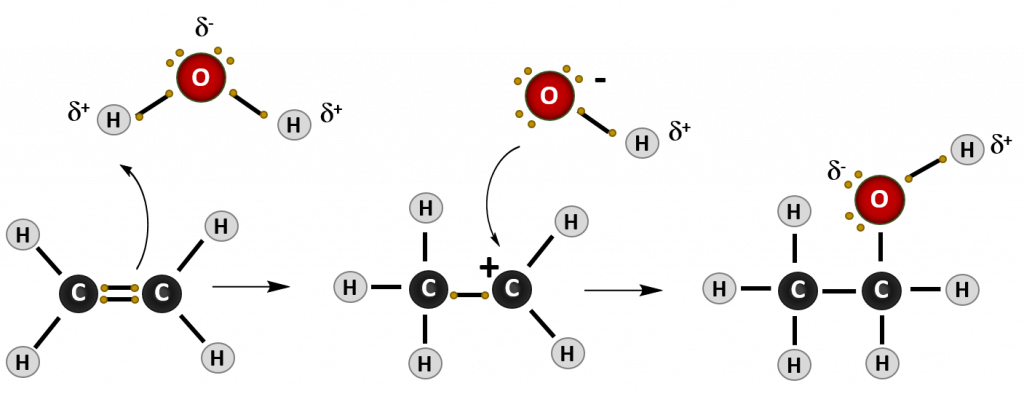
Most reactions that occur with alkenes are addition reactions. As the name implies, during an addition reaction a compound is added to the molecule across the double bond. The result is loss of the double bond (or alkene structure), and the formation of the alkane structure. The reaction mechanism of a reaction describes how the electrons move between molecules to create the chemical reaction. Note that in reaction mechanism diagrams, as shown in Figure 8.15, curved arrows are used to show where electrons are moving. The reaction mechanism for a generic alkene addition equation using the molecule X-Y is shown below:
Figure 8.15. Reaction mechanism of a generic addition reaction. In this reaction, an electron from the carbon-carbon double bond of the alkene attacks an incoming molecule (XY) causing the breakage of the carbon-carbon double bond (lefthand diagram) and formation of a new bond between one of the alkene carbons and molecule X. The original electron from X that was participating in the shared bond with Y, is donated to Y causing the breakage of the X-Y bond. In the intermediate state (middle diagram), the alkene is carrying a positively charged carbon ion, called a carbocation, and Y is in a negatively charged anion state. The negative anion is attracted to the positively charged carbocation and donates the two electrons to form the C-Y bond and complete the product of the addition reaction (righthand diagram).
Key Takeaway:
Addition reactions convert an alkene into an alkane by adding a molecule across the double bond.
There are four major types of addition reactions that can occur with alkenes, they include: Hydogenation, Halogenation, Hydrohalogenation, and Hydration.
1. Hydrogenation
In a Hydrogenation reaction, hydrogen (H2) is added across the double bond, converting an unsaturated molecule into a saturated molecule. Note that the word hydrogen is found in this reaction name, making it easier to remember and recognize: Hydrogen-ation. In a hydrogenation reaction, the final product is the saturated alkane.
2. Halogenation
In a Halogenation reaction group 7A elements (the halogens) are added across the double bond. The most common halogens that are incorporated include chlorine (Cl2), bromine (Br2), and Iodine (I2). Notice that the term halogen is found in this reaction name, making it easier to remember and recognize: Halogen-ation. In halogenation reactions the final product is haloalkane.
3. Hydrohalogenation
In Hydrohalogenation, alkenes react with molecules that contain one hydrogen and one halogen. Hence the name Hydro–Halogen-ation. HCl and HBr are common hydrohalogens seen in this reaction type. In hydrohalogenation, the hydrohalogen is a polar molecule, unlike the nonpolar molecules observed in the halogenation and hydrogenation reactions. In the case of the hydrohalogen, the end of the molecule containing hydrogen is partially positive, while the end of the molecule containing the halogen is partially negative. Thus, when the negatively charged electron from the alkene double bond attacks the hydrohalogen, it will preferentially attack the hydrogen side of the molecule, since the electron will be attracted to the partial positive charge. The halogen will then form the negatively charged anion observed in the intermediate structure and attach second during the addition reaction. The final product is a haloalkane.
4. Hydration
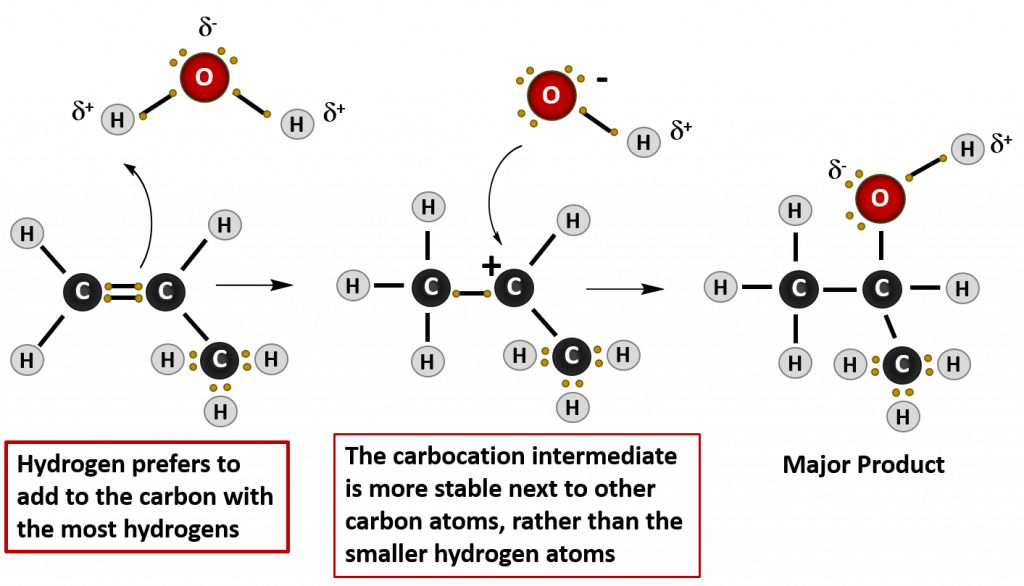
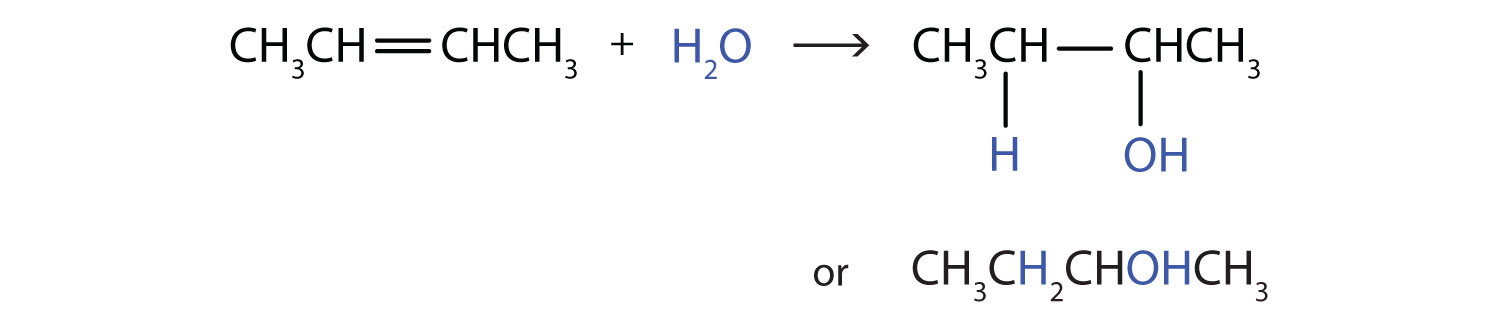
Just like when your are feeling thirsty, the terms hydration and dehydration refer to water. Hydration means the addition of water to a molecule, just like when you feel fully hydrated or full of water, while dehydration means the removal or elimination of water, just as when you are feeling dehydrated and need some water to drink. Similar to the hydrohalogenation reaction above, water is also a polar molecule. In this case, the water is split into two groups to be added across the double bond of the alkene. It is split into the H- and the -OH components. Similar to the hydrohalogenation reaction, the hydrogen adds first, as it carries the partial positive charge. the OH group forms the negative anion intermediate and is then added to the carbocation to form the final product, which is an alcohol.
Markovnikov’s Rule
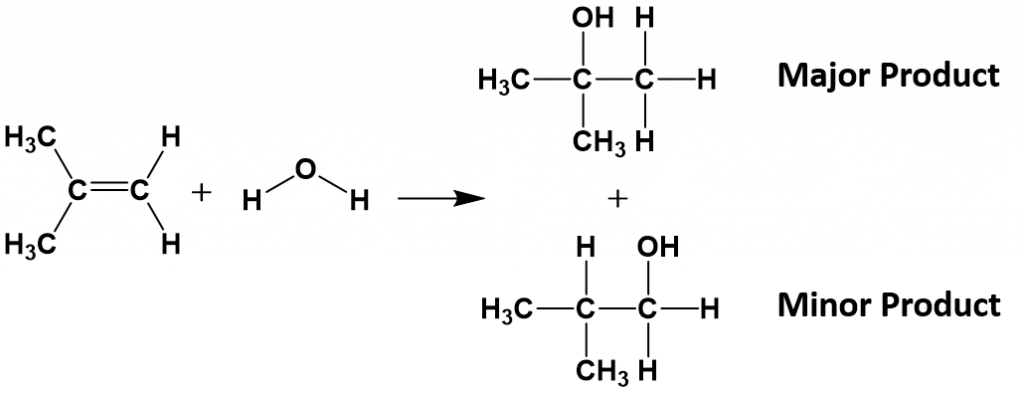
In more complex molecules, hydrohalogenation and hydration reactions can lead the formation of more than one possible product. For example, if 2-methylpropene [(CH3)2CCH2] reacts with water to form the alcohol, two possible products can form, as shown below. However, the addition reaction is not random. One of the products is the major product (being produced in higher abundance) while the other product is the minor product.
In these types of reactions, Markovnikov’s Rule can be used to predict which product will be the major product. Markovnikov’s Rule states that in addition reactions with HX (where X is a halogen or the OH group from water), the H always attaches to the carbon that already has the most hydrogens, and the X attaches the carbon with the fewer hydrogen atoms. This occurs because the carbocation intermediate that forms as the reaction proceeds is more stable when it is bonded to other carbon atoms, than when it is bonded with hydrogen atoms, as seen in the example below:
Extra Practice:
Write the equation for the reaction between CH3CH=CHCH3 and each substance.
- H2
- Br2
- H2O
Solution
In each reaction, the reagent adds across the double bond.
Skill-Building Exercise
Write the equation for each reaction.
-
CH3CH2CH=CH2 with H2 (Ni catalyst)
-
CH3CH=CH2 with Cl2
-
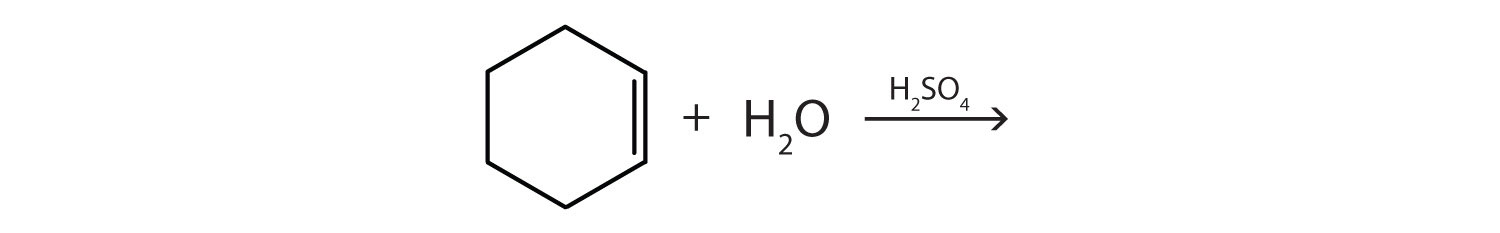
CH3CH2CH=CHCH2CH3 with H2O (H2SO4 catalyst)
Concept Review Exercises
-
What is the principal difference in properties between alkenes and alkanes? How are they alike?
-
If C12H24 reacts with HBr in an addition reaction, what is the molecular formula of the product?
Answers
-
Alkenes undergo addition reactions; alkanes do not. Both burn.
-
C12H24Br2
Key Takeaway
- Alkenes undergo addition reactions, adding such substances as hydrogen, bromine, and water across the carbon-to-carbon double bond.
Exercises
-
Complete each equation.
- (CH3) 2C=CH2 + Br2 →
- CH2=C(CH3)CH2CH3 + H2 →NiCH2=C(CH3)CH2CH3 + H2 →Ni
-

-
Complete each equation.
- CH2=CHCH=CH2 + 2H2→NiCH2=CHCH=CH2 + 2H2→Ni
- (CH3)2C=C(CH3)2 + H2O →(CH3)2C=C(CH3)2 + H2O →H2SO4
-

Answer
-
- (CH3)2CBrCH2Br
- CH3CH(CH3)CH2CH3
-

(Back to the Top)
Elimination Reactions
In an elimination reaction a molecule loses a functional group, typically a halogen or an alcohol group, and a hydrogen atom from two adjacent carbon atoms to create an alkene structure. Elimination reactions are essentially the reverse reaction of the hydration and hydrohalogenation addition reactions. Elimination reactions can also occur with the removal of water from alcohol
Rearrangement Reactions
A rearrangement reaction is a specific organic reaction that causes the alteration of the structure to form an isomer. With alkene structures, rearrangement reactions often result in the conversion of a cis-isomer into the trans conformation.
Substitution Reactions
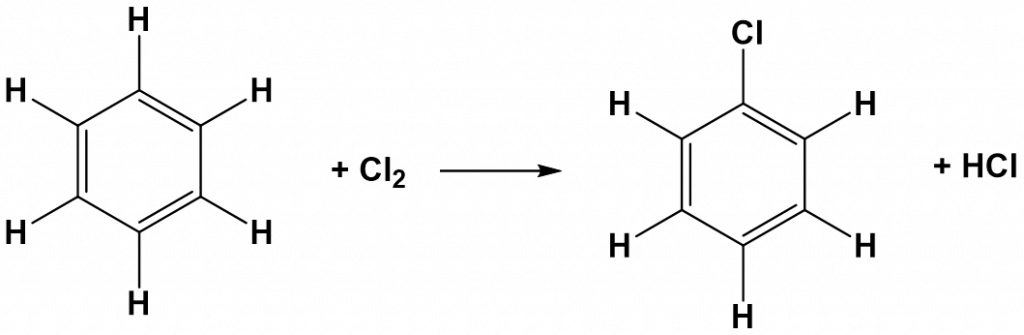
Due to the high reactivity of alkenes, they usually undergo addition reactions rather than substitutions reactions. The exception is the benzene ring. The double-bonded structure of the benzene ring gives this molecule a resonance structure such that all of the carbon atoms in the ring share a continually rotating partial bond structure.
Thus, the overall structure is very stable compared to other alkenes and benzene rings do not readily undergo addition reactions. They behave more similarly to alkane structure and lack chemical reactivity. One of the few types of reactions that a benzene ring will undergo is a substitution reaction. Recall from Chapter 7 that in substitution reactions an atom or group of atoms is replaced by another atom or group of atoms. Halogenation is a common substitution reaction that occurs with benzene ring structures. In the diagram below, notice that the hydgrogen atom is substituted by one of the bromine atoms.
(Back to the Top)
8.7 Alkene Polymers
The most important commercial reactions of alkenes are polymerizations, reactions in which small molecules, referred to in general as monomers, (from the Greek monos, meaning “one,” and meros, meaning “parts”), are assembled into giant molecules referred to as polymers (from the Greek poly, meaning “many,” and meros, meaning “parts”). A polymer is as different from its monomer as a long strand of spaghetti is from a tiny speck of flour. For example, polyethylene, the familiar waxy material used to make plastic bags, is made from the monomer ethylene—a gas.
The Production of Polyethene
click to see the Royal Society of Chemistry Video on Polyethene Production
Polyethene pellets that are produced in factories can be melted, formed into a giant bubble, and then made into a film that is used in packaging, consumer products, and food services.
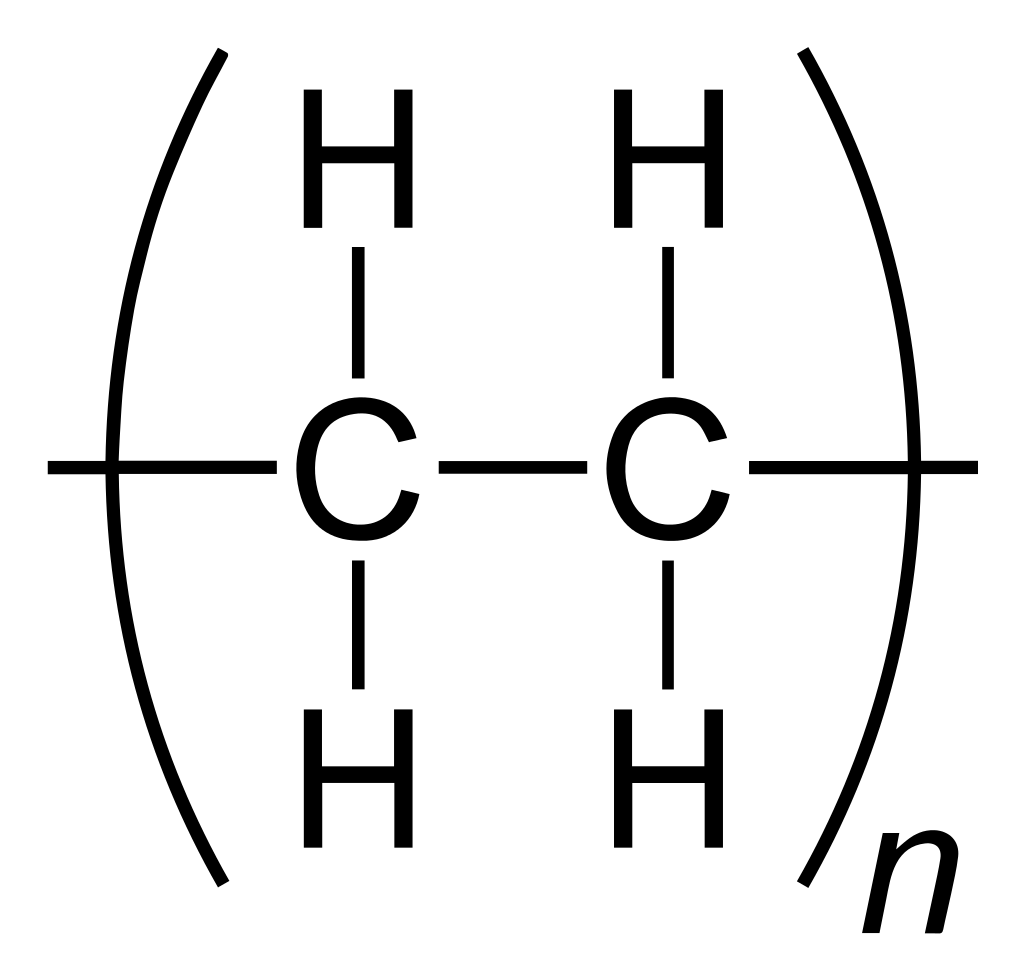
There are two general types of polymerization reactions: addition polymerization and condensation polymerization. This section will focus on addition polymerization reactions. (For more information about condensation polymerization, see Chapter 10) In addition polymerization, the monomers add to one another in such a way that the polymer contains all the atoms of the starting monomers. Ethylene molecules are joined together in long chains. The polymerization can be represented by the reaction of a few monomer units:
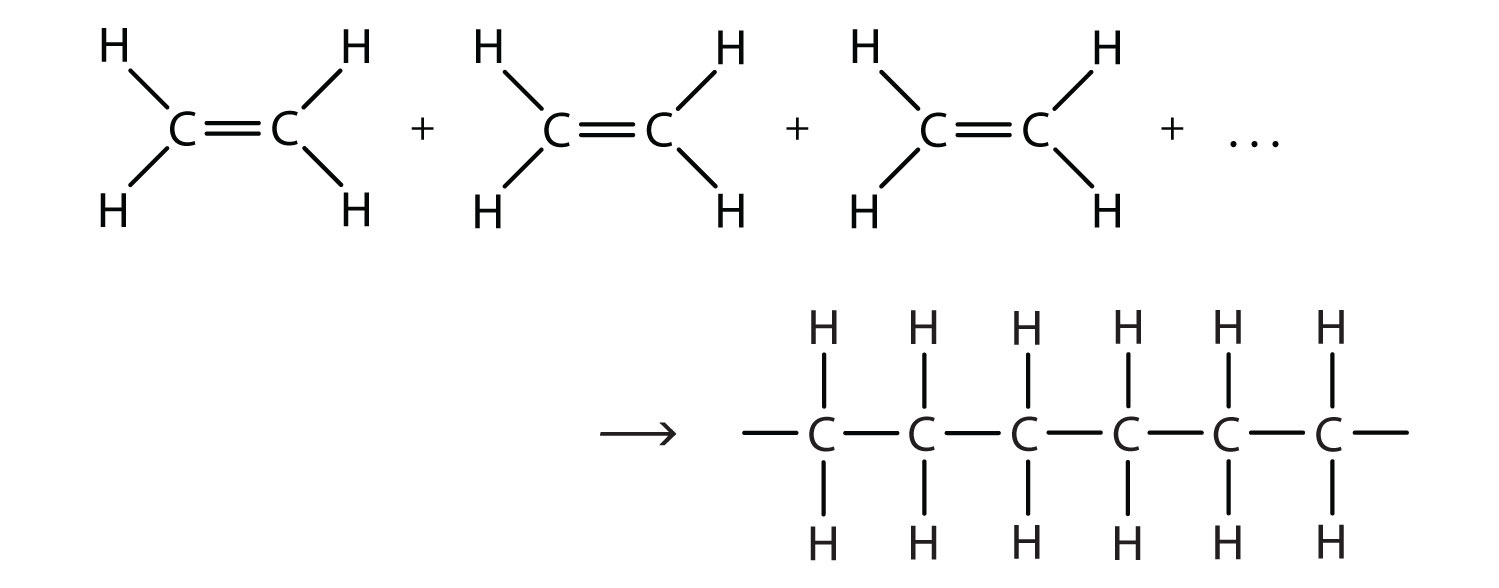
The bond lines extending at the ends in the formula of the product indicate that the structure extends for many units in each direction. Notice that all the atoms—two carbon atoms and four hydrogen atoms—of each monomer molecule are incorporated into the polymer structure. Because displays such as the one above are cumbersome, the polymerization is often abbreviated as follows, where n is the number of repeating units:
Structure from: Magmar452
Note
Many natural materials—such as proteins, cellulose and starch, and complex silicate minerals—are polymers. Artificial fibers, films, plastics, semisolid resins, and rubbers are also polymers. More than half the compounds produced by the chemical industry are synthetic polymers.
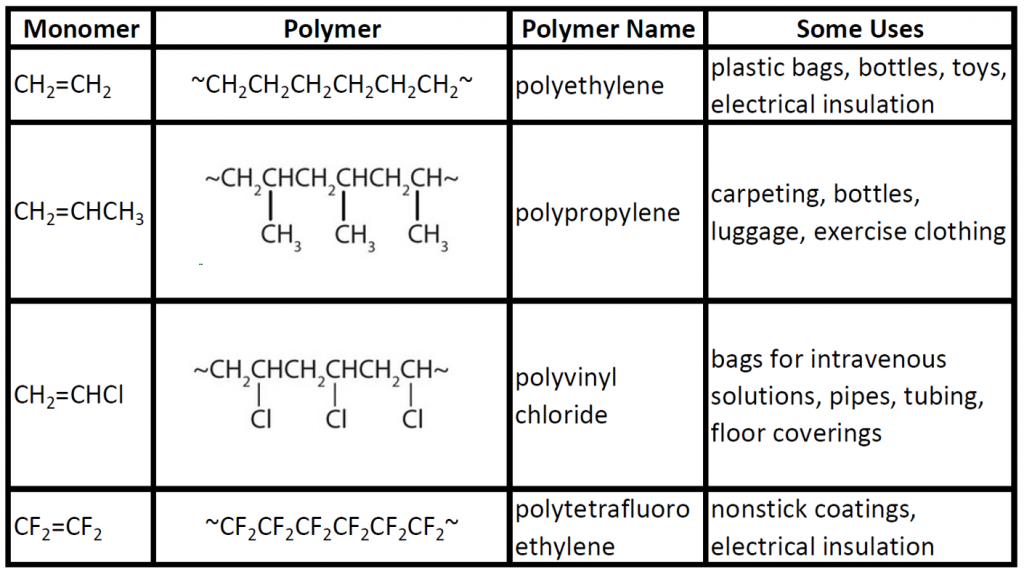
Some common addition polymers are listed in Table 8.2. Note that all the monomers have carbon-to-carbon double bonds. Many polymers are mundane (e.g., plastic bags, food wrap, toys, and tableware), but there are also polymers that conduct electricity, have amazing adhesive properties, or are stronger than steel but much lighter in weight.
Medical Uses of Polymers
An interesting use of polymers is the replacement of diseased, worn out, or missing parts in the body. For example, about a 250,000 hip joints and 500,000 knees are replaced in US hospitals each year. The artificial ball-and-socket hip joints are made of a special steel (the ball) and plastic (the socket). People crippled by arthritis or injuries gain freedom of movement and relief from pain. Patients with heart and circulatory problems can be helped by replacing worn out heart valves with parts based on synthetic polymers. These are only a few of the many biomedical uses of polymers.
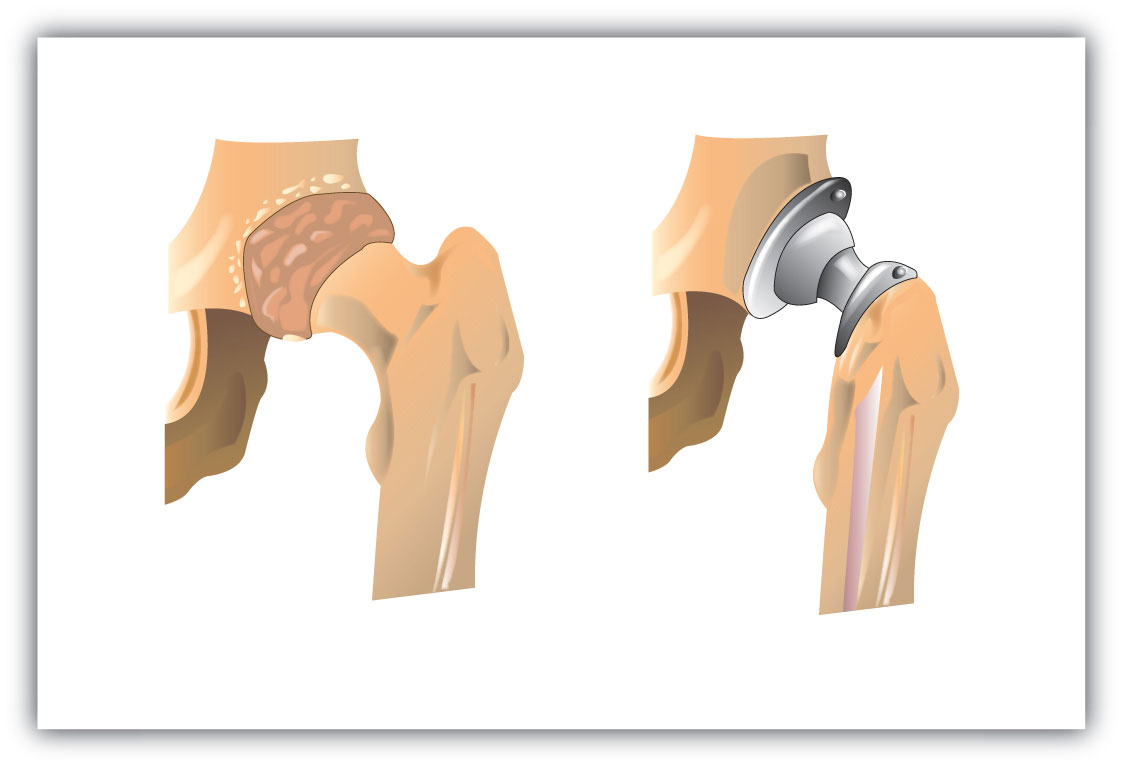
Figure 8.16 Hip Joint Replacement Synthetic polymers are an important part of a hip joint replacement. The hip is much like a ball-and-socket joint, and total hip replacements mimic this with a metal ball that fits in a plastic cup.
Concept Review Exercises
-
What is a monomer? What is a polymer? How do polymer molecules differ from the molecules we have discussed in earlier sections of this chapter?
-
What is addition polymerization? What structural feature usually characterizes molecules used as monomers in addition polymerization?
-
What is the molecular formula of a polymer molecule formed by the addition polymerization of 175 molecules of vinyl chloride (CH2=CHCl)?
Answers
-
Monomers are small molecules that can be assembled into giant molecules referred to as polymers, which are much larger than the molecules we discussed earlier in this chapter.
-
In addition polymerization, the monomers add to one another in such a way that the polymer contains all the atoms of the starting monomers.
-
C350H525Cl175
Key Takeaway
- Molecules having carbon-to-carbon double bonds can undergo addition polymerization.
Exercises
-
Write the condensed structural formula of the monomer from which Saran is formed. A segment of the Saran molecule has the following structure: CH2CCl2CH2CCl2CH2CCl2CH2CCl2.
-
Write the condensed structural formula for the section of a molecule formed from four units of the monomer CH2=CHF.
Answer
-
H2C=CCl2
(Back to the Top)
8.8 Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
Any hydrocarbon containing either a double or triple bond is an unsaturated hydrocarbon. Alkenes have a carbon-to-carbon double bond. The general formula for alkenes with one double bond is CnH2n. Alkenes can be straight chain, branched chain, or cyclic. Simple alkenes often have common names, but all alkenes can be named by the system of the International Union of Pure and Applied Chemistry and have the ending -ene.
Cis-trans isomers (or geometric isomers) are characterized by molecules that differ only in their configuration around a rigid part of the structure, such as a carbon–to-carbon double bond or a ring. The molecule having two identical (or closely related) atoms or groups on the same side is the cis isomer; the one having the two groups on opposite sides is the trans isomer.
The physical properties of alkenes are quite similar to those of alkanes. Like other hydrocarbons, alkenes are insoluble in water but soluble in organic solvents.
More reactive than alkanes, alkenes undergo Addition Reactions across the double bond. There are four types of addition reactions: Hydrogenation which involves adding H2 across the double bond, Hydrohalogenation which involves adding hydrogen and a halogen (Cl, Br, or I) across the double bond, Halogenation which involves adding two halogen atoms (Cl, Br, or I) across the double bond, and Hydration which involves adding water (as H and -OH) across the double bond. Alkenes also undergo addition polymerization, molecules joining together to form long-chain molecules.
…CH2=CH2 + CH2=CH2 + CH2=CH2 +…→…CH2CH2–CH2CH2–CH2CH2–…
The reactant units are monomers, and the product is a polymer.
Alkenes can also be involved in Rearrangement Reactions that convert one compound into a related isomer. Rearranging cis to trans isomers are common rearrangement reactions. Elimination Reactions can regenerate alkene structures by the removal of water or dehydration of alkanes.
Alkynes have a carbon-to-carbon triple bond. The general formula for alkynes is CnH2n − 2. The properties of alkynes are quite similar to those of alkenes. They are named much like alkenes but with the ending –yne.
Aromatic compounds contain a cyclic hydrocarbon, benzene (C6H6) with alternating double-bonds. Due to resonance structures, the aromatic ring is extremely stable and does not undergo the typical reactions expected of alkenes. The electrons that might be fixed in three double bonds are instead delocalized over all six carbon atoms. The main reaction aromatic compounds will undergo are substitution reactions. A polycyclic aromatic hydrocarbon (PAH) has fused benzene rings sharing a common side.
Additional Exercises
-
Classify each compound as saturated or unsaturated.
-

- CH3C≡CCH3
-
-
Classify each compound as saturated or unsaturated.
-
-
Give the molecular formula for each compound.
-
-
Describe a physiological effect of some PAHs.
-
What are some of the hazards associated with the use of benzene?
-
What is wrong with each name?
- 2-bromobenzene
- 3,3-dichlorotoluene
- 1,4-dimethylnitrobenzene
-
Following are line-angle formulas for three compounds. Draw the uncondensed structure for each.
-
(Back to the Top)
8.9 References
Text for this chapter has been adapted from the creative commons resources listed below, unless otherwise noted in the text.
- Organic Chemistry (2016) Libretexts, U.C. Davis, Licenced under: Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License. Available at: https://chem.libretexts.org/Core/Organic_Chemistry
- Inhalant. (2017, February 12). In Wikipedia, The Free Encyclopedia. Retrieved 01:21, February 13, 2017, from https://en.wikipedia.org/w/index.php?title=Inhalant&oldid=765135147
- Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
- Physical and Theoretical Chemistry (2017) Libretexts, U.C. Davis, Licenced under: Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Bond_Energies.
- Petroleum. (2017, February 14). In Wikipedia, The Free Encyclopedia. Retrieved 06:29, February 16, 2017, from https://en.wikipedia.org/w/index.php?title=Petroleum&oldid=765440823
- Ball, D.W., Hill, J.W., and Scott, R.J. (2016) MAP: The Basics of General, Organic and Biological Chemistry. Libre Texts. Available at: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry_Textbook_Maps/Map%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)