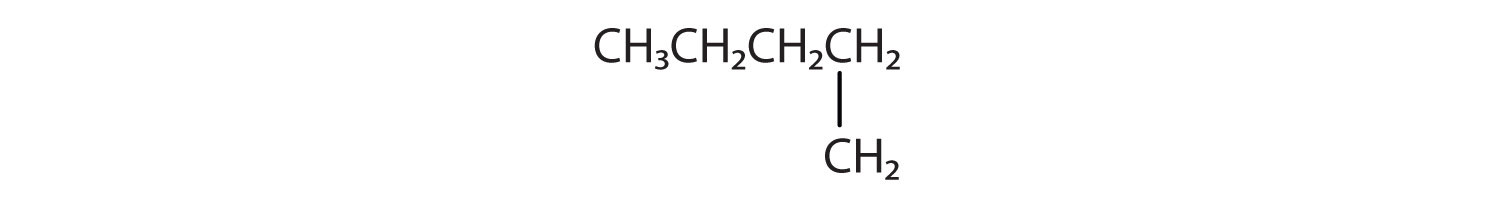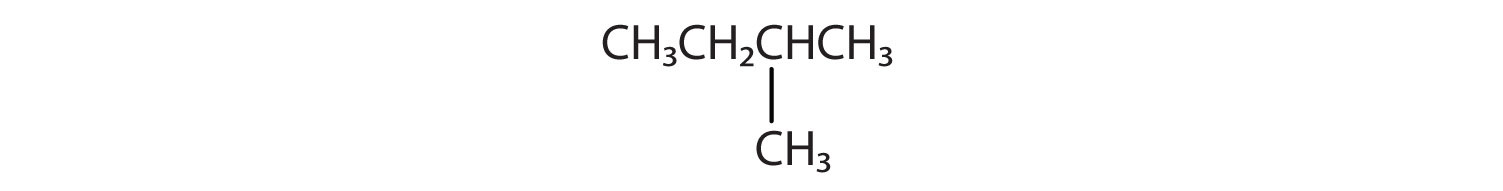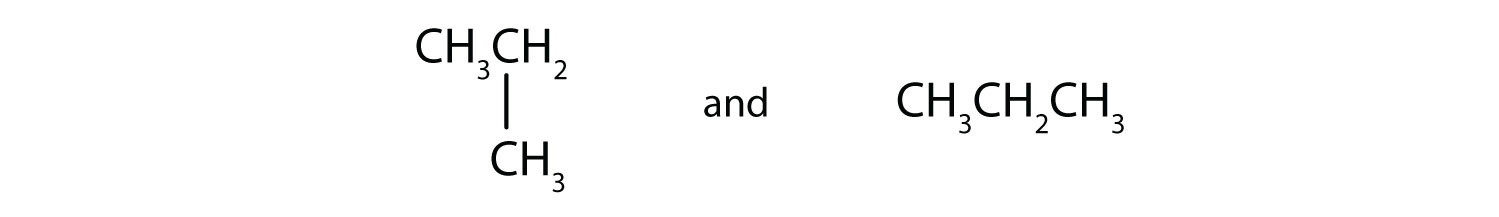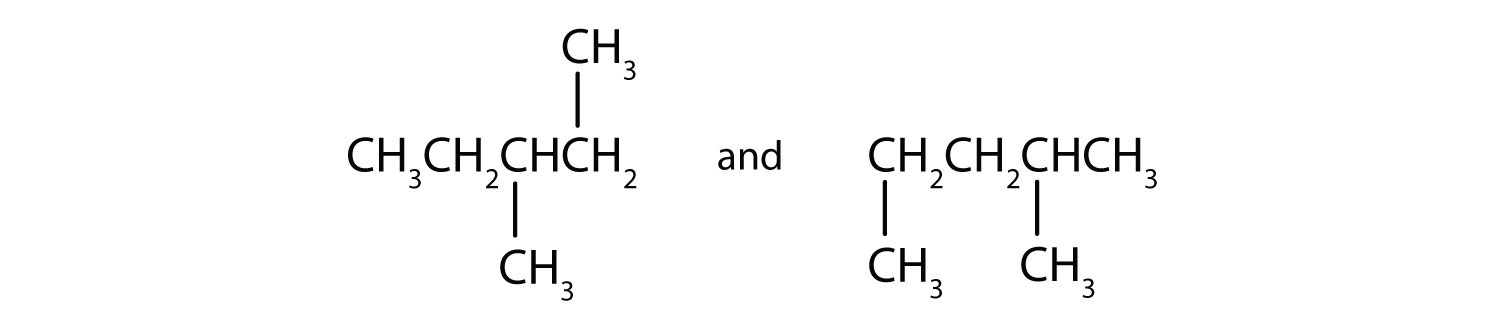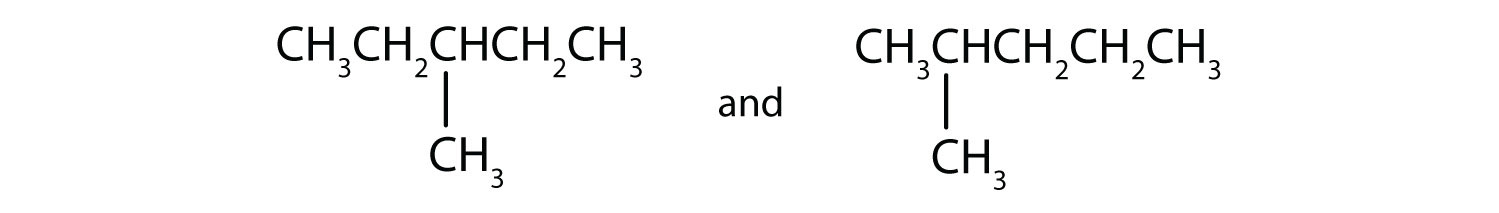Home » Student Resources » Online Chemistry Textbooks » CH105: Consumer Chemistry » CH105: Chapter 7 – Alkanes and Halogenated Hydrocarbons
MenuCH105: Consumer Chemistry
Chapter 7: Alkanes and Halogenated Hydrocarbons
This text is published under creative commons licensing, for referencing and adaptation, please click here.
Opening Essay
7.1 Recognition of Organic Structures
7.2 Introduction to Alkanes
Straight Chain Alkanes
Branched Chain Alkanes
Cycloalkanes
Classification of Carbon Bonds
7.3 Properties of Alkanes
Melting Points and Boiling Points
Solubility
Alkane Properties and Environmental Hazards: A Closer Look
7.4 Chemical Reactivity of Alkanes
Combustion Reactions
Halogenation Reactions (Substitution Type)
Cracking Alkanes
7.5 Chapter Summary
7.6 End-of-Chapter Exercises
7.7 References
Opening Essay
Hydrocarbons are the simplest organic compounds, but they have interesting physiological effects. These effects depend on the size of the hydrocarbon molecules and where on or in the body they are applied. Alkanes of low molar mass—those with from 1 to approximately 10 or so carbon atoms—are gases or light liquids that act as anesthetics. Inhaling (“sniffing”) these hydrocarbons in gasoline or aerosol propellants for their intoxicating effect is a major health problem that can lead to liver, kidney, or brain damage or to immediate death by asphyxiation by excluding oxygen. Pressurized canisters of propane and butane gas, both of which are intended for use as fuels, are abused as inhalants.
Figure 7.1. A range of petroleum-based products that can be abused as inhalants. Photo By: Lance Cpl. Matthew K. Hacker
Swallowed, liquid alkanes do little harm while in the stomach. In the lungs, however, they cause “chemical” pneumonia by dissolving fatlike molecules from cell membranes in the tiny air sacs (alveoli). The lungs become unable to expel fluids, just as in pneumonia caused by bacteria or viruses. People who swallow gasoline or other liquid alkane mixtures should not be made to vomit, as this would increase the chance of getting alkanes into the lungs. (There is no home-treatment antidote for gasoline poisoning; call a poison control center.)
Liquid alkanes with approximately 5–16 carbon atoms per molecule wash away natural skin oils and cause drying and chapping of the skin, while heavier liquid alkanes (those with approximately 17 or more carbon atoms per molecule) act as emollients (skin softeners). Such alkane mixtures as mineral oil and petroleum jelly can be applied as a protective film. Water and aqueous solutions such as urine will not dissolve such a film, which explains why petroleum jelly protects a baby’s tender skin from diaper rash.
In this chapter we will investigate the alkanes, compounds containing only two elements, carbon and hydrogen, and having only single bonds. We will also investigate alkanes that have halogens incorporated into their structure. Recall that halogens are the elements in Family 7A on the periodic table and contain representative elements such as chlorine, fluorine, iodine, and bromine. There are several other kinds of hydrocarbons, distinguished by the types of bonding between carbon atoms and by the properties that result from that bonding. In Chapter 8 we will examine hydrocarbons with double bonds, with triple bonds, and with a special kind of bonding called aromaticity. Then in Chapter 9, we will study some compounds considered to be derived from hydrocarbons by replacing one or more hydrogen atoms with an oxygen-containing group. Chapter 10 focuses on organic acids and bases.
(Back to the Top)
7.1 Recognition of Organic Structures
Learning Objective
- To be able to recognize the composition and properties typical of organic and inorganic compounds.
Scientists of the 18th and early 19th centuries studied compounds obtained from plants and animals and labeled them organic because they were isolated from “organized” (living) systems. Compounds isolated from nonliving systems, such as rocks and ores, the atmosphere, and the oceans, were labeled inorganic. For many years, scientists thought organic compounds could be made by only living organisms because they possessed a vital force found only in living systems. The vital force theory began to decline in 1828, when the German chemist Friedrich Wöhler synthesized urea from inorganic starting materials. He reacted silver cyanate (AgOCN) and ammonium chloride (NH4Cl), expecting to get ammonium cyanate (NH4OCN). What he expected is described by the following equation.
AgOCN + NH4Cl → AgCl + NH4OCN
Instead, he found the product to be urea (NH2CONH2), a well-known organic material readily isolated from urine. This result led to a series of experiments in which a wide variety of organic compounds were made from inorganic starting materials. The vital force theory gradually went away as chemists learned that they could make many organic compounds in the laboratory.
Today organic chemistry has been reclassified as the study of compounds that contain carbon, and inorganic chemistry is the study of the chemistry of all other elements. It may seem strange that we divide chemistry into two branches—one that considers compounds of only one element and one that covers the 100-plus remaining elements. However, this division seems more reasonable when we consider that of tens of millions of compounds that have been characterized, the overwhelming majority are carbon compounds.
Note
The word organic has different meanings. Organic fertilizer, such as cow manure, is organic in the original sense; it is derived from living organisms. Organic foods generally are foods grown without synthetic pesticides or fertilizers. Organic chemistry is the chemistry of compounds of carbon. Carbon is unique among the other elements in that its atoms can form stable covalent bonds with each other and with atoms of other elements in a multitude of variations. The resulting molecules can contain from one to millions of carbon atoms.
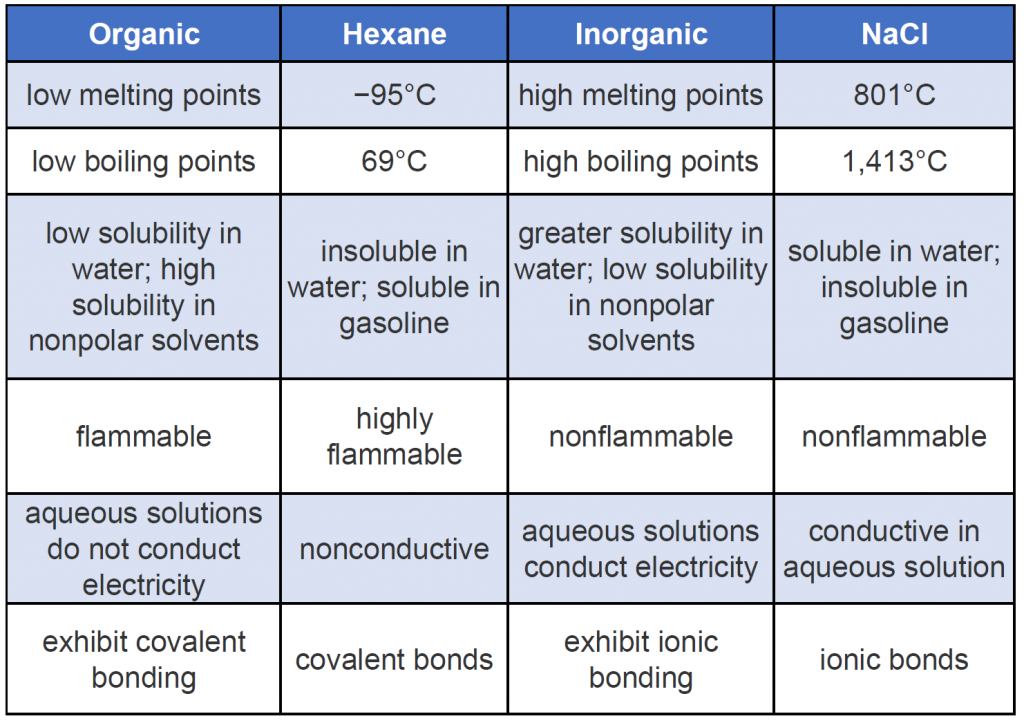
Organic compounds, like inorganic compounds, obey all the natural laws. Often there is no clear distinction in the chemical or physical properties among organic and inorganic molecules. Nevertheless, it is useful to compare typical members of each class, as in Table 7.1 (Keep in mind, however, that there are exceptions to every category in this table.) To further illustrate typical differences among organic and inorganic compounds, Table 7.1 also lists properties of the inorganic compound sodium chloride (common table salt, NaCl) and the organic compound hexane (C6H14), a solvent that is used to extract soybean oil from soybeans (among other uses). Many compounds can be classified as organic or inorganic by the presence or absence of certain typical properties, as illustrated in Table 7.1.
Table 7.1 General Contrasting Properties and Examples of Organic and Inorganic Compounds
Concept Review Exercises: Click to Complete the Questions
Key Takeaway
- Organic chemistry is the study of carbon compounds, nearly all of which also contain hydrogen atoms.
More Practice
-
Classify each compound as organic or inorganic.
- C6H10
- CoCl2
- C12H22O11
-
Classify each compound as organic or inorganic.
- CH3NH2
- NaNH2
- Cu(NH3)6Cl2
-
Which member of each pair has a higher melting point?
- CH3OH and NaOH
- CH3Cl and KCl
-
Which member of each pair has a higher melting point?
- C2H6 and CoCl2
- CH4 and LiH
Answers to odd questions:
-
- organic
- inorganic
- organic
-
- NaOH
- KCl
(Back to the Top)
7.2 Introduction to Alkanes
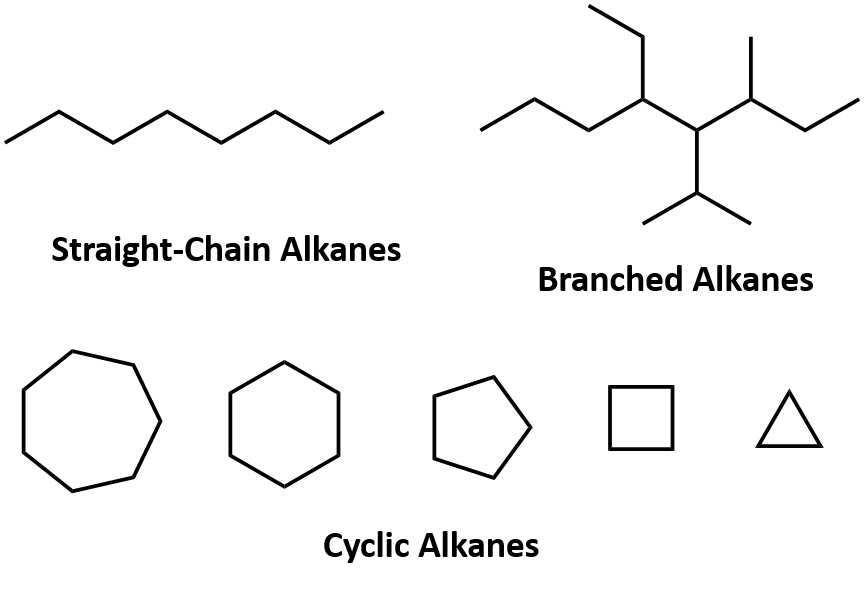
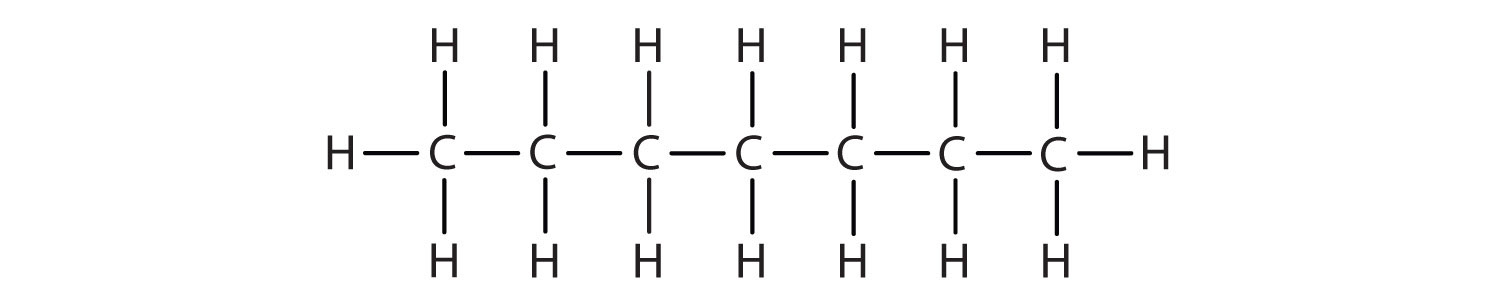
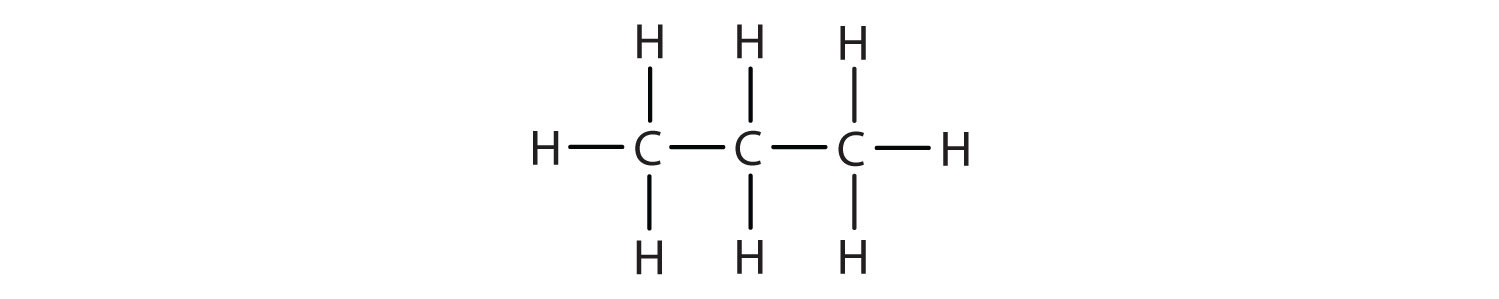
Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkanes have the general formula CnH2n+2 and can be subdivided into the following three groups: the linear straight-chain alkanes, branched alkanes, and cycloalkanes (Fig. 7.2). Alkanes are also saturated hydrocarbons, that is all of the carbon atoms are ‘saturated’ with hydrogen atoms and do not contain any carbon-carbon double bonds or triple bonds. Alkanes are the simplest and least reactive hydrocarbon species containing only carbons and hydrogens. They are commercially very important, being the principal constituent of gasoline and lubricating oils and are extensively employed in organic chemistry; though the role of pure alkanes (such as hexanes) is delegated mostly to solvents. The distinguishing feature of an alkane, making it distinct from other compounds that also exclusively contain carbon and hydrogen, is its lack of unsaturation. That is to say, it contains no double or triple bonds, which are highly reactive in organic chemistry. Though not totally devoid of reactivity, their lack of reactivity under most laboratory conditions makes them a relatively uninteresting, though very important component of organic chemistry. As you will learn about later, the energy confined within the carbon-carbon bond and the carbon-hydrogen bond is quite high and their rapid oxidation produces a large amount of heat, typically in the form of fire.
Figure 7.2. Examples of Alkanes
Straight Chain Alkanes
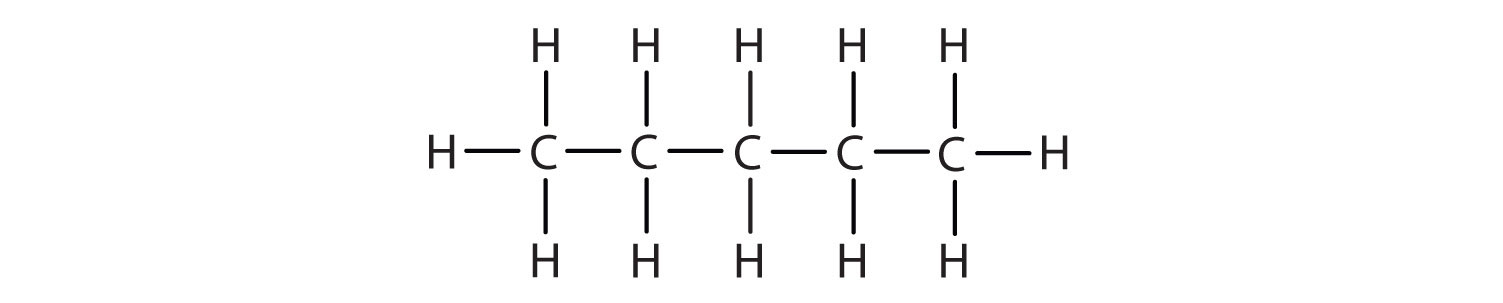
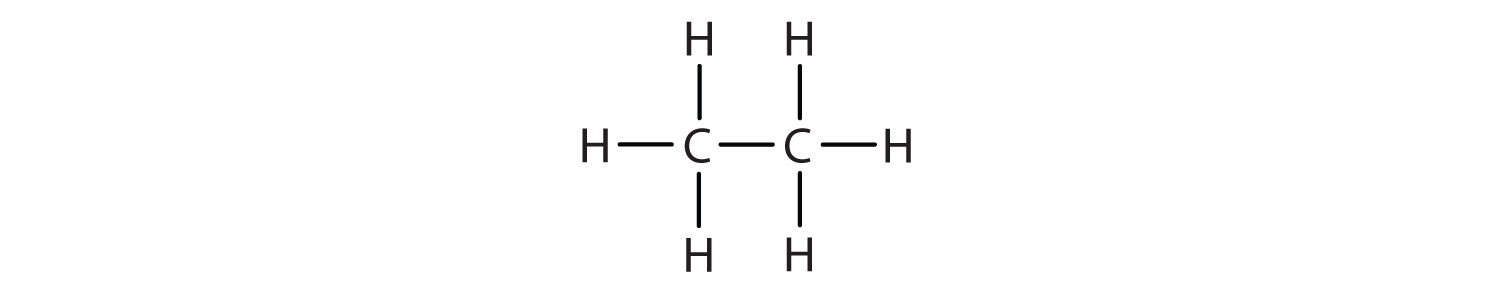
The straight chain alkanes, methane (CH4), ethane (C2H6), and propane (C3H8) represent the beginning of a series of compounds in which any two members in a sequence differ by one carbon atom and two hydrogen atoms—namely, a CH2 unit (Fig. 7.3)
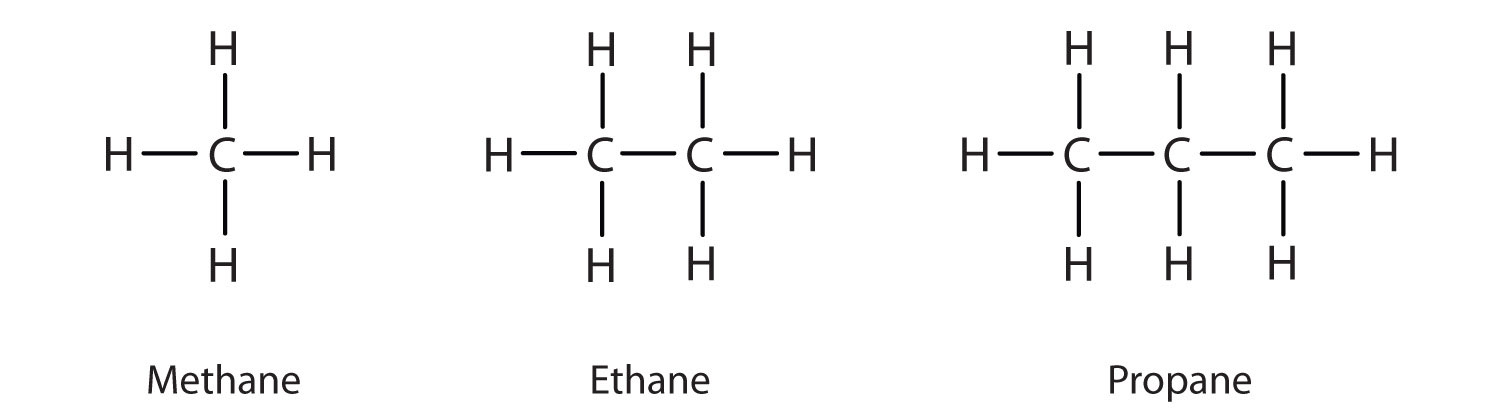
Figure 7.3 The Three Simplest Alkanes
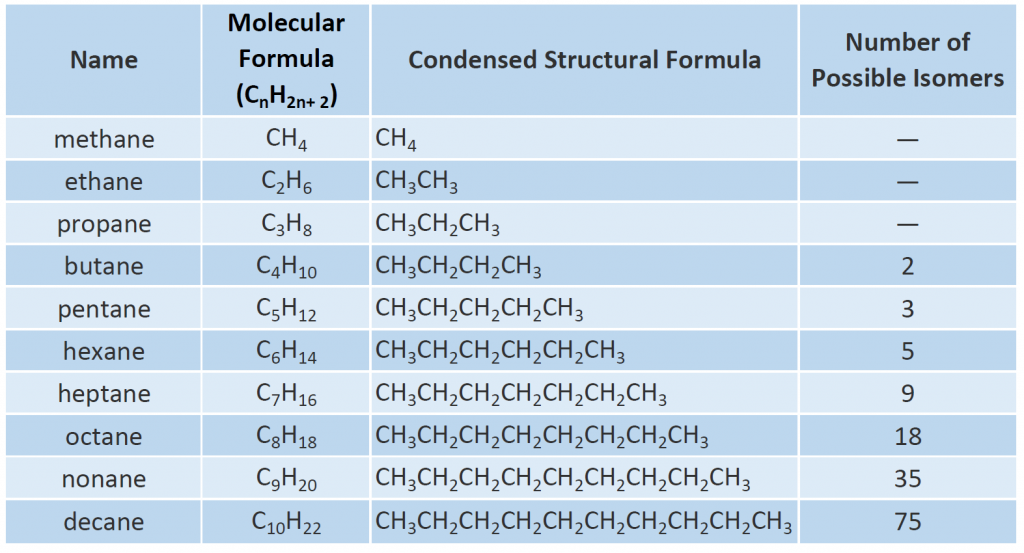
The first 10 members of this series are given in Table 7.2. Note that as you increase the length of the carbon chain, the number of possible different structural isomers also increases.
From propane (C3H8) onward, you will notice that the only difference between longer chain hydrocarbons involves the addition of CH2 units as you move up the series (Fig. 7.4). Any family of compounds in which adjacent members differ from each other by a definite factor (here a CH2 group) is called a homologous series, and can be defined mathematically. The members of such a series, called homologs. In organic chemistry, homologs have properties that vary in a regular and predictable manner. Thus, the principle of homology gives organization to organic chemistry in much the same way that the periodic table gives organization to inorganic chemistry. Instead of a bewildering array of individual carbon compounds, we can study a few members of a homologous series and from them deduce some of the properties of other compounds in the series.
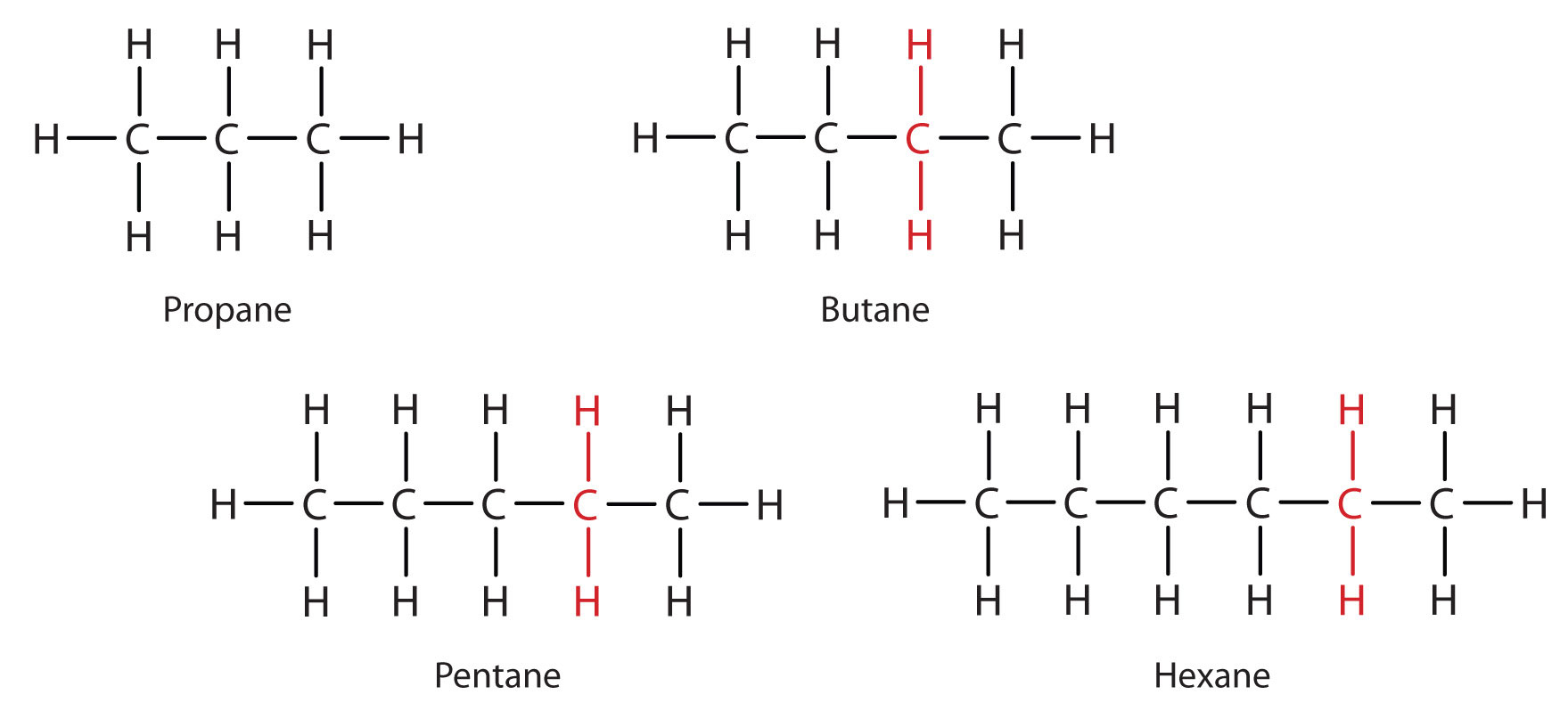
Figure 7.4 Members of a Homologous Series
In Figure 7.4, note that each succeeding formula incorporates one carbon atom and two hydrogen atoms more than the previous formula. The principle of homology allows us to write a general formula for alkanes: CnH2n+ 2. Using this formula, we can write a molecular formula for any alkane with a given number of carbon atoms. For example, an alkane with eight carbon atoms has the molecular formula C8H(2 × 8) + 2 = C8H18.
Concept Review Exercises
-
In the homologous series of alkanes, what is the molecular formula for the member just above C8H18?
-
Use the general formula for alkanes to write the molecular formula of the alkane with 12 carbon atoms.
Answers
-
C9H20
-
C12H26
(Back to the Top)
Branched Chain Alkanes
We can write the structure of butane (C4H10) by stringing four carbon atoms in a row,
–C–C–C–C–
and then adding enough hydrogen atoms to give each carbon atom four bonds:
The compound butane has this structure, but there is another way to put 4 carbon atoms and 10 hydrogen atoms together. Place 3 of the carbon atoms in a row and then branch the fourth one off the middle carbon atom:
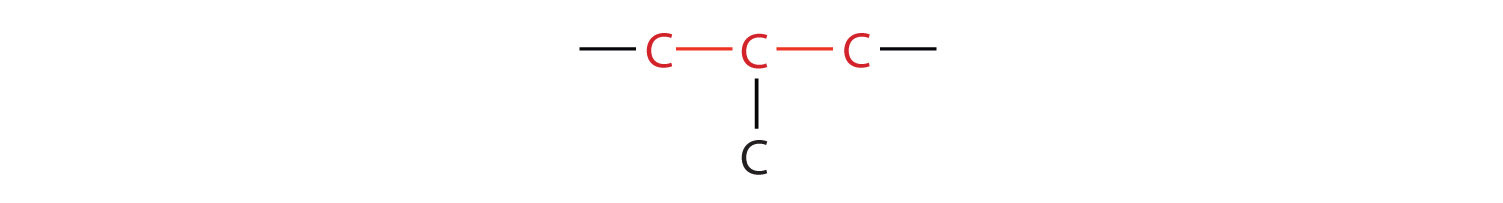
Now we add enough hydrogen atoms to give each carbon four bonds.
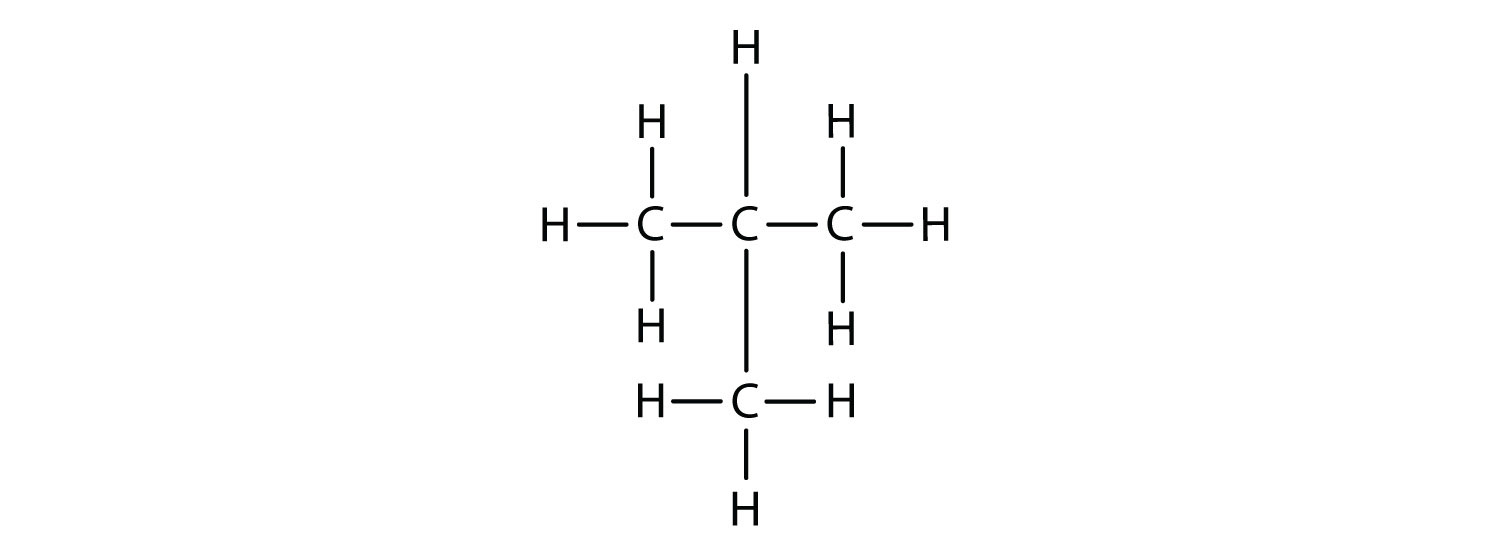
There is a hydrocarbon that corresponds to this structure, which means that two different compounds have the same molecular formula (C4H10), but a different arrangement of the atoms in space. Recall that compounds having the same molecular formula but a different arrangement in space are called structural isomers. Structural isomers have different chemical and physical properties. Figure 7.5 shows the ball and stick model for the straight chain butane and the branched isomer, isobutane.
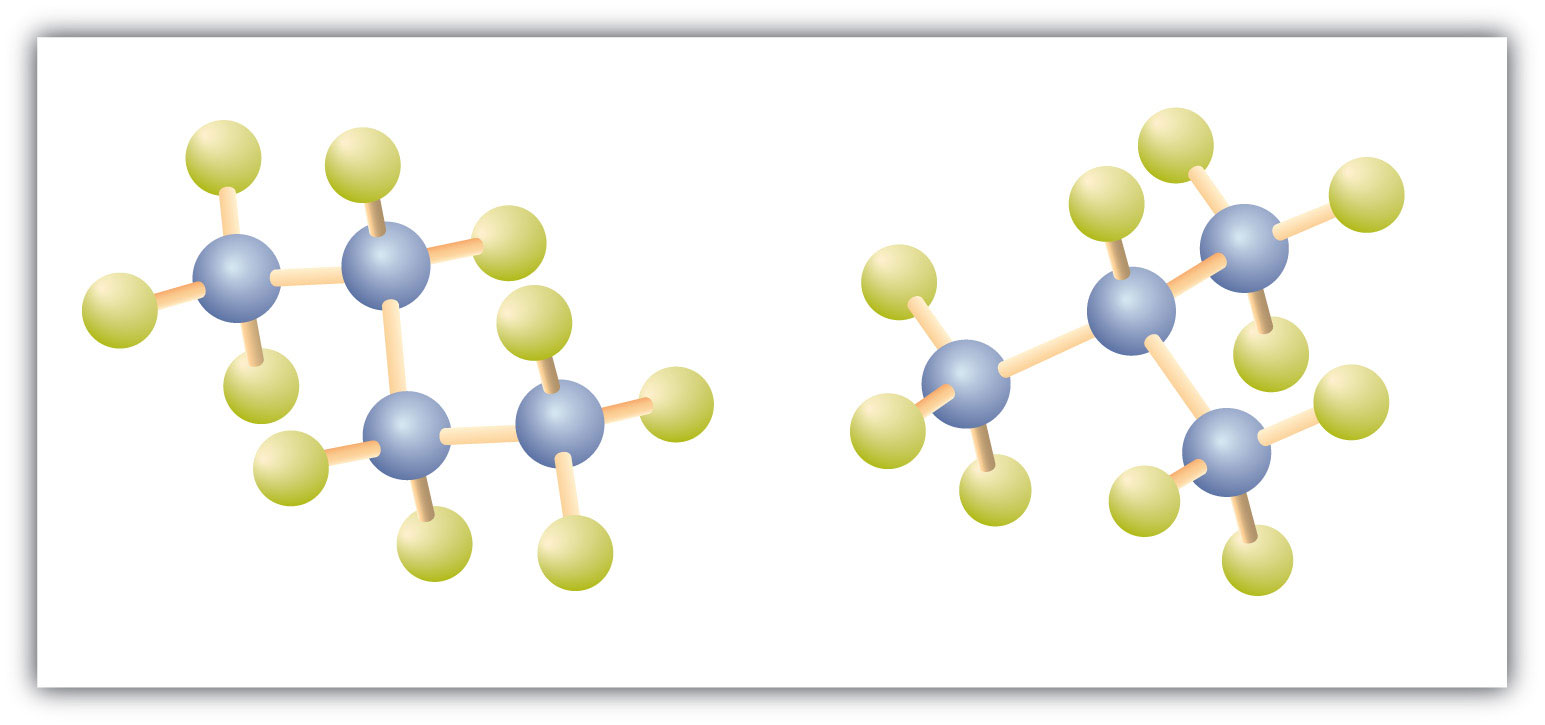
Figure 7.5 Butane and Isobutane. The ball-and-stick models of these two compounds show them to be isomers; both have the molecular formula C4H10.
Notice that C4H10 is depicted with a bent chain in Figure 7.5. The four-carbon chain may be bent in various ways because the groups can rotate freely about the C–C bonds. However, this rotation does not change the identity of the compound. It is important to realize that bending a chain does not change the identity of the compound; all of the following represent the same compound:
The formula of isobutane shows a continuous chain of three carbon atoms only, with the fourth attached as a branch off the middle carbon atom of the continuous chain.
Unlike C4H10, the compounds methane (CH4), ethane (C2H6), and propane (C3H8) do not exist in isomeric forms because there is only one way to arrange the atoms in each formula so that each carbon atom has four bonds.
Note
A continuous (unbranched) chain of carbon atoms is often called a straight chain even though the tetrahedral arrangement about each carbon gives it a zigzag shape. Straight-chain alkanes are sometimes called normal alkanes, and their names are given the prefix n-. For example, butane is called n-butane.
Concept Review Exercises
-
In alkanes, can there be a two-carbon branch off the second carbon atom of a four-carbon chain? Explain.
-
A student is asked to write structural formulas for two different hydrocarbons having the molecular formula C5H12. She writes one formula with all five carbon atoms in a horizontal line and the other with four carbon atoms in a line, with a CH3 group extending down from the first attached to the third carbon atom. Do these structural formulas represent different molecular formulas? Explain why or why not.
Answers
-
No; the branch would make the longest continuous chain of five carbon atoms.
-
No; both are five-carbon continuous chains.
Key Takeaway
- Alkanes with four or more carbon atoms can exist in isomeric forms.
Exercises
-
Briefly identify the important distinctions between a straight-chain alkane and a branched-chain alkane.
-
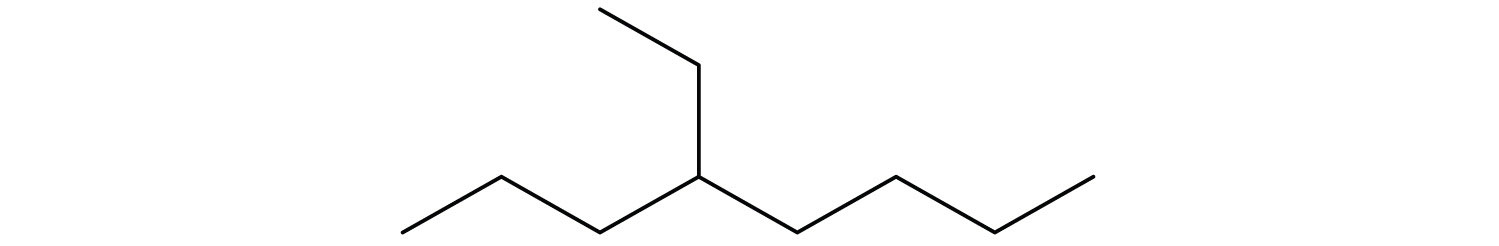
Draw the structural isomers for the following alkanes.
-
-
Indicate whether the structures in each set represent the same compound or isomers.
-
CH3CH2CH2CH3 and

-
CH3CH2CH2CH2CH3 and

-
Answers
-
Straight-chain alkanes and branched-chain alkanes have different properties as well as different structures.
-
- no
- yes
Additional Practice
-
Write the condensed structural formula for each structural formula.
-
-
A condensed structural formula for isohexane can be written as (CH3)2CHCH2CH2CH3. Draw the line-angle formula for isohexane.
-
Draw a line-angle formula for the compound CH3CH2CH(CH3)CH2CH2CH3.
-
Give the structural formula for the compound represented by this line-angle formula:

(Back to the Top)
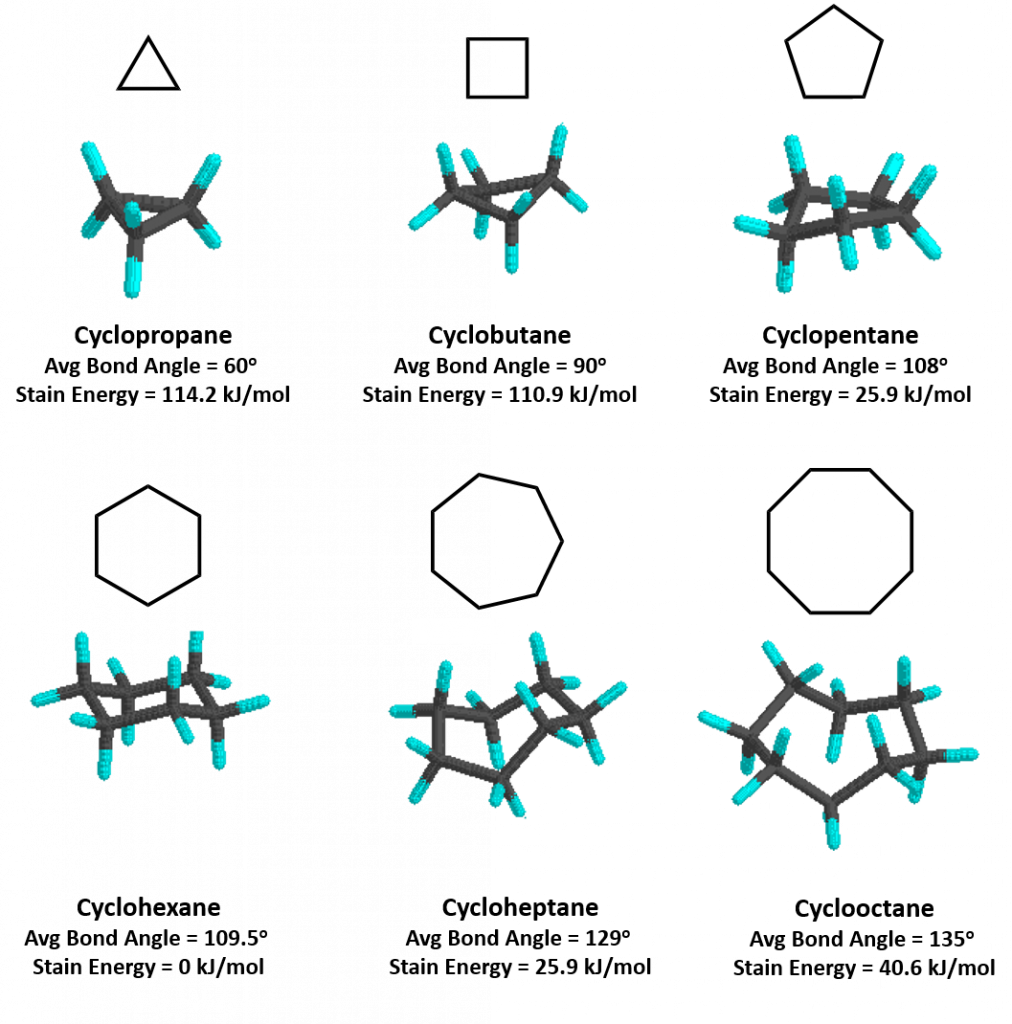
Cycloalkanes are very important in components of food, pharmaceutical drugs, and much more. However, to use cycloalkanes in such applications, we must know the effects, functions, properties, and structures of cycloalkanes. Cycloalkanes are alkanes that are in the form of a ring; hence, the prefix cyclo- is used to name these alkanes. Stable cycloalkanes cannot be formed with carbon chains of just any length. Recall that in alkanes, carbon adopts the tetrahedral geometry in which the angles between bonds are 109.5°.
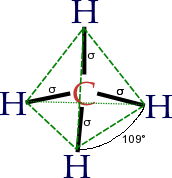 Source: Wikipedia
Source: Wikipedia
For some cycloalkanes to form, the angle between bonds must deviate from this ideal angle, an effect known as angle strain. Additionally, some hydrogen atoms may come into closer proximity with each other than is desirable (become eclipsed), an effect called torsional strain. These destabilizing effects, angle strain and torsional strain are known together as ring strain. The smaller cycloalkanes, cyclopropane and cyclobutane, have particularly high ring strains because their bond angles deviate substantially from 109.5° and their hydrogens eclipse each other. Thus, both of these ring conformations are highly unfavorable and unstable. Cyclopentane is a more stable molecule with a small amount of ring strain, while cyclohexane is able to adopt the perfect geometry of a cycloalkane in which all angles are the ideal 109.5° and no hydrogens are eclipsed; it has no ring strain at all. Cycloalkanes larger than cyclohexane have ring strain and are not as commonly encountered in organic chemistry. Figure 7.6 provides examples of cycloalkane structures.
Figure 7.6. Representative Cycloalkane Structures. Average bond angles and strain energy are indicated.
To Your Health: Cyclopropane as an Anesthetic
With its boiling point of −33°C, cyclopropane is a gas at room temperature. It is also a potent, quick-acting anesthetic with few undesirable side effects in the body. It is no longer used in surgery, however, because it forms explosive mixtures with air at nearly all concentrations.
(Back to the Top)
Classification of Carbon Bonds
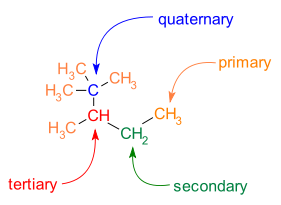
Carbon atoms participating in chemical bonds within a molecule can be classified based on the number of carbon-carbon bonds that are formed.
- primary carbon atom: one carbon neighbor
- secondary carbon atom: two carbon neighbors
- tertiary carbon atom: three carbon neighbors
- quaternary carbon atom: four carbon neighbors
The number of carbon neighbors that a carbon atom has can help determine the reactivity of that carbon position. Thus, it is important to be able to recognize whether a carbon atom is primary, secondary, tertiary, or quaternary in its structure (Fig. 7.7).
Figure 7.7. Classification of carbon atoms as primary, secondary, tertiary, or quaternary. In the molecules above, the center carbon is evaluated for the number of carbon atoms that are bonded directly with the center carbon. A primary carbon is bonded to one carbon, a secondary carbon is bonded to two carbons, a tertiary carbon is bonded to three carbons, and a quaternary carbon is bonded to four carbons.
Within any given molecule, each carbon atom can be classified (Fig. 7.8).
Figure 7.8. Classification of Carbon Atoms Within a Molecule
(Back to the Top)
7.3 Properties of Alkanes
Alkanes are the simplest family of hydrocarbons – compounds containing carbon and hydrogen only with only carbon-hydrogen bonds and carbon-carbon single bonds. Alkanes are not very reactive and have little biological activity; all alkanes are colorless and odorless. Because alkanes have relatively predictable physical properties and undergo relatively few chemical reactions other than combustion, they serve as a basis of comparison for the properties of many other organic compound families. Let’s consider their physical properties first.
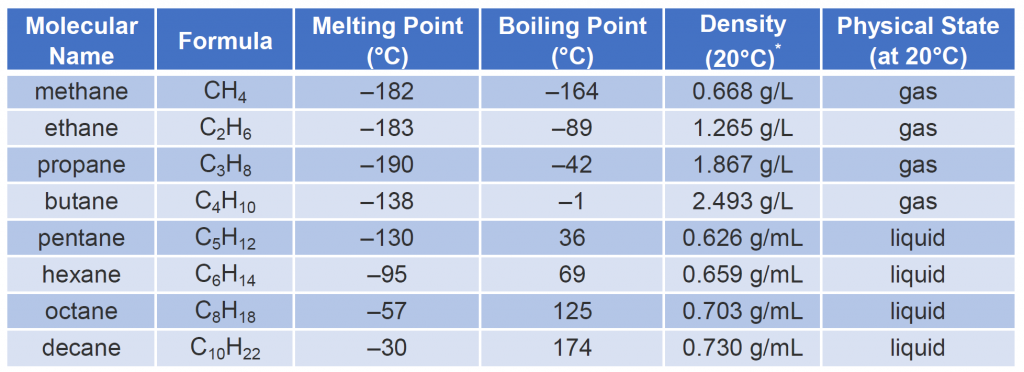
Table 7.3 describes some of the properties of some of the first 10 straight-chain alkanes. Nearly all alkanes have densities less than 1.0 g/mL and are therefore less dense than water (the density of H2O is 1.00 g/mL at 20°C).
Looking Closer: Gas Densities and Fire Hazards
Table 7.3 indicates that the first four members of the alkane series are gases at ordinary temperatures. Natural gas is composed chiefly of methane, which has a density of about 0.67 g/L. The density of air is about 1.29 g/L. Because natural gas is less dense than air, it rises. When a natural-gas leak is detected and shut off in a room, the gas can be removed by opening an upper window. On the other hand, bottled gas can be either propane (density 1.88 g/L) or butanes (a mixture of butane and isobutane; density about 2.5 g/L). Both are much heavier than air (density 1.2 g/L). If bottled gas escapes into a building, it collects near the floor. This presents a much more serious fire hazard than a natural-gas leak because it is more difficult to rid the room of the heavier gas.
(Back to the Top)
Melting Points and Boiling Points
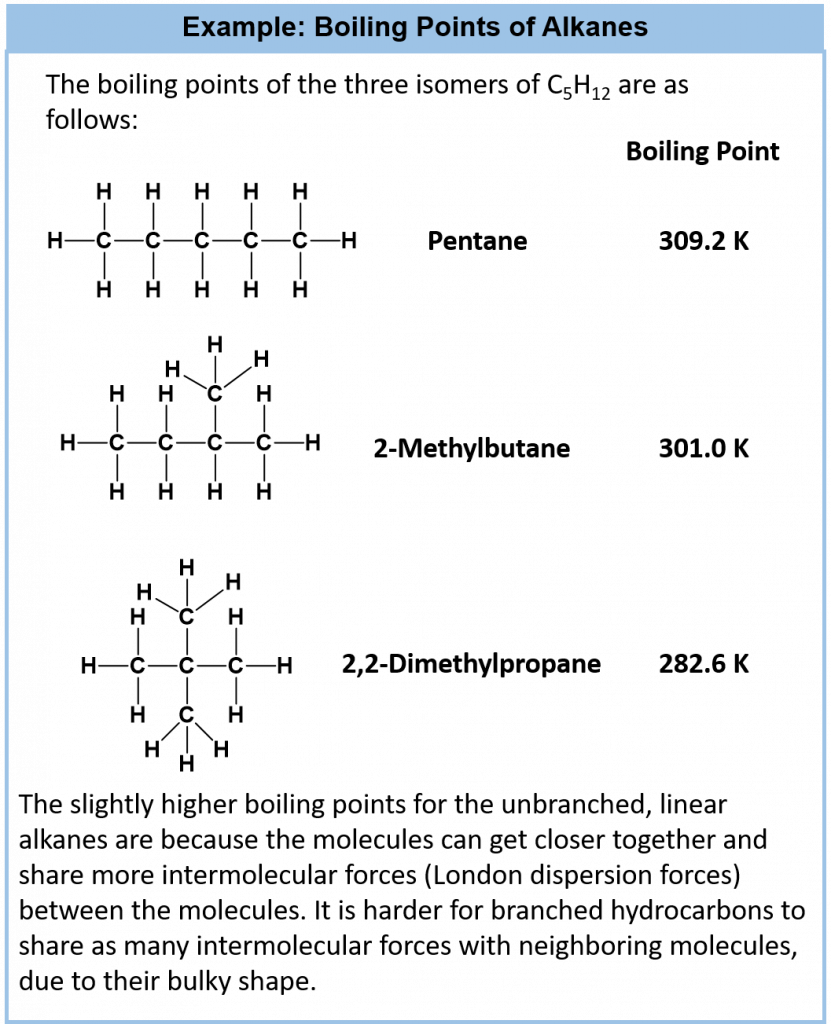
Both the melting points and boiling points of alkanes are characteristic of the intermolecular forces found between the molecules. The electronegativity difference between carbon and hydrogen (2.1 – 1.9 = 0.2) is small; therefore, the C-H bond is nonpolar, meaning that the only attractions between one molecule and its neighbors will be London dispersion forces. These forces will be very small for a molecule like methane but will increase as the size of the molecules increase. Therefore, the melting and boiling points of the alkanes increases with the molecular size, due to the increase in London dispersion forces. (i.e. the intermolecular forces are stronger in larger hydrocarbons, therefore, more energy is required to cause phase changes). Figure 7.9 shows the melting and boiling point trends for the first 16 hydrocarbons. Notice that the first four alkanes are gases at room temperature, and solids do not start to appear until about C17H36.
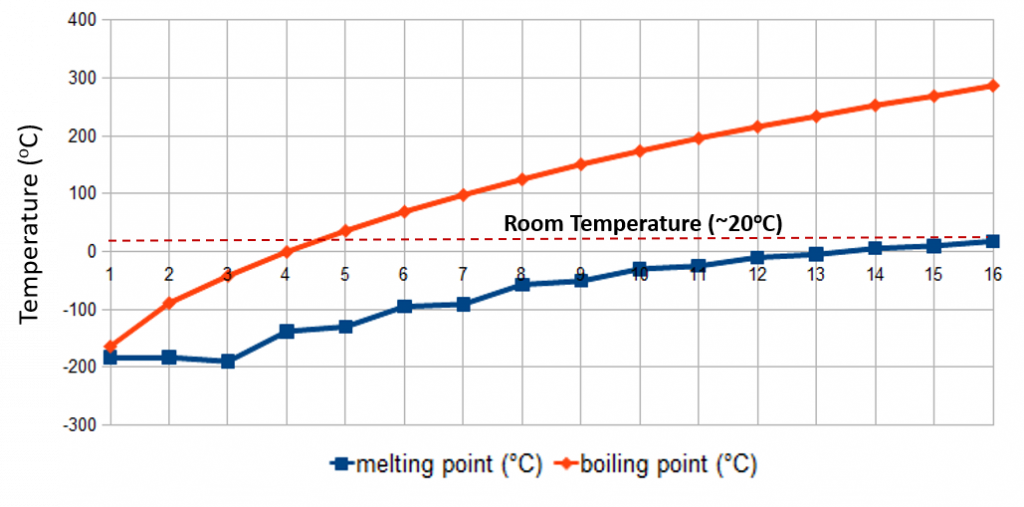
Figure 7.9. Melting Points and Boiling Points for Straight Chain Alkanes
Adapted from: Techstepp
Regarding isomers, the more branched the chain, the lower the boiling point tends to be. London dispersion forces are smaller for shorter molecules and only operate over very short distances between one molecule and its neighbors. It is more difficult for short, bulky molecules (with substantial amounts of branching) to lie close together (compact) compared with long, thin molecules. Cycloalkanes are similar to alkanes in their general physical properties, but they have higher boiling points, melting points, and densities than alkanes. This is due to stronger London forces because the ring shape allows for a larger area of contact.
(Back to the Top)
Solubility
Alkanes (both normal and cycloalkanes) are virtually insoluble in water but dissolve in organic solvents. The liquid alkanes are good solvents for many other covalent compounds. When a molecular substance dissolves in water, the following must occur:
- breaking of the intermolecular forces within the substance. In the case of the alkanes, these are the London dispersion forces.
- breaking of the intermolecular forces in the water so that the substance can fit between the water molecules. In water, the primary intermolecular attractions are hydrogen bonds.
Breaking either of these attractions requires energy, although the amount of energy required to break the London dispersion forces in a compound, such as methane, is relatively negligible; this is not true of the hydrogen bonds in water. Recall that hydrogen bonds are much stronger.
To simplify, a substance will dissolve if sufficient energy is released when the new bonds are formed between the substance and the water to make up for the energy required to break the original attractions. The only new attractions between the alkane and the water molecules are the London dispersion forces. These forces to do not release a sufficient amount of energy to compensate for the energy required to break the hydrogen bonds in water. Therefore, the alkane does not dissolve as shown in Figure 7.10.
Figure 7.10. Long Chain Hydrocarbons are Insoluble in Water. Neither the saturated and unsaturated hydrocarbons in this sunflower oil do not have strong enough intermolecular forces to disrupt the hydrogen bonds between the water molecules. Thus, the oil is not soluble in the water and forms beaded bubbles of oil at the surface of the water/oil interface. In this case the oil is less dense than the water and will float on top of the water layer.
Solubility in organic solvents
In most organic solvents, the primary forces of attraction between the solvent molecules are the London dispersion forces. Therefore, when an alkane dissolves in an organic solvent, the London dispersion forces are broken and are replaced by new London dispersion forces between the mixture. The two processes more or less cancel each other out energetically; thus, there is no barrier to solubility.
(Back to the Top)
Alkane Properties and Environmental Hazards: A Closer Look
Due to the solubility and density of alkanes, oil spills into the ocean or other bodies of water can have devastating environmental consequences. The oil cannot dissolve or mix with the water, and because it is less dense than water, it floats on top of the surface of the water creating an oil slick, as depicted in Figure 7.11. Since the oil slick remains at the surface of the water, the organisms most affected by oil slicks are those found on the surface of the ocean or near the shorelines, including sea otters and seabirds. The chemical constituents of the oil are toxic though ingestion, inhalation, and through skin and eye irritation.

Figure 7.11 Oil Spills. Crude oil coats the water’s surface in the Gulf of Mexico after the Deepwater Horizon oil rig sank following an explosion. The leak was a mile below the surface, making it difficult to estimate the size of the spill. One liter of oil can create a slick 2.5 hectares (6.3 acres) in size. This and similar spills provide a reminder that hydrocarbons and water don’t mix.
Source: Photo courtesy of NASA Goddard / MODIS Rapid Response Team.
Concept Review Exercises
-
If 25 mL of hexane were added to 100 mL of water in a beaker, which of the following would you expect to happen? Explain.
- Hexane would dissolve in water.
- Hexane would not dissolve in water and would float on top.
- Hexane would not dissolve in water and would sink to the bottom of the container.
Answers
-
dodecane because of its greater molar mass
-
b; hexane is insoluble in water and less dense than water.
Key Takeaway
- Alkanes are nonpolar compounds that are low boiling and insoluble in water.
Exercises
-
For which member of each pair is hexane a good solvent?
- pentane or water
- sodium chloride or soybean oil
Answer
-
- pentane
- nonane
(Back to the Top)
7.4 Chemical Reactivity of Alkanes
Alkanes contain strong carbon-carbon single bonds and strong carbon-hydrogen bonds. Both of these bonds are nonpolar. Therefore, there is no portion of the molecule that carries any significant amount of positive or negative charge, which is required for other ionic and polar molecules to be attracted to it. Therefore alkanes generally do not react with ionic compounds such as most laboratory acids, bases, oxidizing agents, or reducing agents. Consider butane as an example:
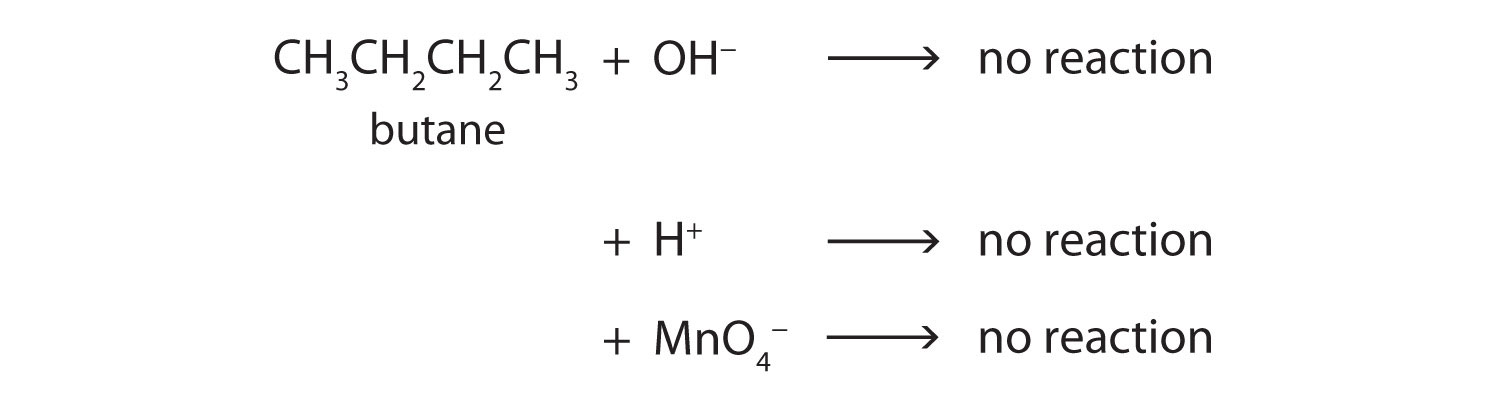
Neither positive ions nor negative ions are attracted to a nonpolar molecule. In fact, the alkanes undergo so few reactions that they are sometimes called paraffins, from the Latin parum affinis, meaning “little affinity.”
The result is that alkanes have very little reactivity and only undergo three major types of reactions, including the following:
- Combustion Reactions – burn them – destroying the entire molecule;
- Halogenation Reactions (substitution type) – react them with some of the halogens, breaking the carbon-hydrogen bonds;
- Cracking Reactions – use heat and/or a catalyst to crack alkanes, breaking carbon-carbon bonds.
Combustion Reactions
The combustion of carbon compounds, especially hydrocarbons, has been the most important source of heat energy for human civilizations throughout recorded history. The practical importance of this reaction cannot be denied, but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. Using the combustion of propane as an example, we see from the following equation that every covalent bond in the reactants has been broken and an entirely new set of covalent bonds have formed in the products. No other common reaction involves such a profound and pervasive change, and the mechanism of combustion is so complex that chemists are just beginning to explore and understand some of its elementary features.
Note that in the reaction above, that the compounds on the left side of the arrow are called the substrates or reactants and the compounds on the right side of the arrow are the products of the reaction. Energy, most often released as heat, can either be a substrate or the product of the reaction, depending on the compounds involved. Also note that none of the atoms are lost within a given chemical equations. The Law of Conservation of Mass states that matter can neither be created or destroyed. Thus, the equation must be balanced and have the same number of each atom on the left side of the equation as on the right. Recall that the coefficients of the equation determine how many moles of compound are present, and that if no coefficient is shown, it is one by default. For example, the equation above would be read as: 1 mole of propane (CH3CH2CH3) reacts with 5 moles of oxygen (O2) to produce 3 moles of carbon dioxide (CO2) and 4 moles of water (H2O) and heat is released.
Chemical bonds also hold potential energy. If you think about the nature of a bond, it is held together by the energy of attraction. To break apart bonds on the reactant side of the equation requires energy, while the formation of bonds on the product side will release energy. If the energy released by bond formation is higher than that required for bond dissociation, energy will be released by the reaction. This energy is usually measured as heat of the reaction, which is called enthalpy.
Two points concerning this reaction are important:
- Since all the covalent bonds in the reactant molecules are broken, the quantity of heat evolved in this reaction is related to the strength of these bonds (and, of course, the strength of the bonds formed in the products). These heats can be evaluated using the bond dissociation/formation energies (the energy required to break a bond on the reactant-side of the equation and the energy released by bond formation on the product-side of the equation).
- The stoichiometry of the reactants is also important. If insufficient oxygen is supplied some of the products will consist of the less oxidized carbon monoxide (CO) gas.
For example, if only 4 moles of oxygen was present, the combustion of one mole of propane would produce only 1 mole of carbon dioxide and 2 moles of carbon monoxide.
Bond Energies
Atoms bond together to form compounds because in doing so they attain lower energies than they possess as individual atoms. A quantity of energy, equal to the difference between the energies of the bonded atoms and the energies of the separated atoms, is released, usually as heat. That is, the bonded atoms have a lower energy than the individual atoms have as separate atoms. When atoms combine to make a compound, energy is always given off, and the compound has a lower overall energy.
When a chemical reaction occurs, molecular bonds are broken and other bonds are formed to make different molecules. For example, the bonds of two water molecules are broken to form hydrogen and oxygen.
Energy is always required to break a bond, which is known as the bond energy. While the concept may seem simple, bond energy serves a very important purpose in describing the structure and characteristics of a molecule. It can be used to determine which Lewis Dot Structure is most suitable when there are multiple Lewis Dot Structures.
Key Takeaways:
- Energy is always required to break a bond.
- Energy is always released when a bond is made.
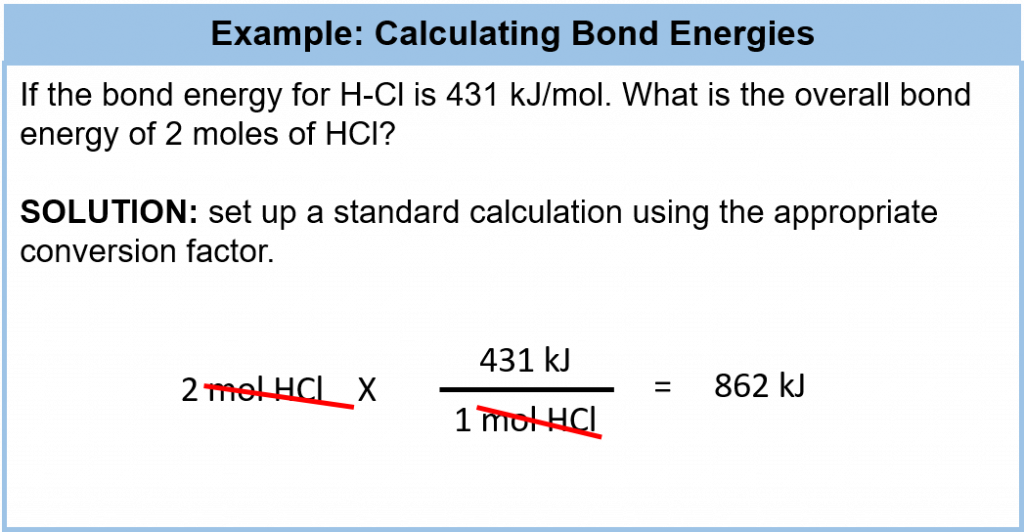
Although each molecule has its own characteristic bond energy, some generalizations are possible. For example, although the exact value of a C–H bond energy depends on the particular molecule, all C–H bonds have a bond energy of roughly the same value because they are all C–H bonds. It takes roughly 100 kcal of energy to break 1 mol of C–H bonds, so we speak of the bond energy of a C–H bond as being about 100 kcal/mol. A C–C bond has an approximate bond energy of 80 kcal/mol, while a C=C has a bond energy of about 145 kcal/mol. We can calculate a more general bond energy by finding the average of the bond energies of a specific bond in different molecules to get the average bond energy. Table 7.2 provides a list of average bond energies for common organic bonds. Remember that energy can be evaluated in kcal or kJ and that the conversion factor between the two is: 4.184 kJ = 1 kcal
| Single Bonds | Multiple Bonds | ||||||
|---|---|---|---|---|---|---|---|
| H—H |
432
|
N—H |
391
|
I—I |
149
|
C = C |
614
|
| H—F |
565
|
N—N |
160
|
I—Cl |
208
|
C ≡ C |
839
|
| H—Cl |
427
|
N—F |
272
|
I—Br |
175
|
O = O |
495
|
| H—Br |
363
|
N—Cl |
200
|
C = O* |
745
|
||
| H—I |
295
|
N—Br |
243
|
S—H |
347
|
C ≡ O |
1072
|
| N—O |
201
|
S—F |
327
|
N = O |
607
|
||
| C—H |
413
|
O—H |
467
|
S—Cl |
253
|
N = N |
418
|
| C—C |
347
|
O—O |
146
|
S—Br |
218
|
N ≡ N |
941
|
| C—N |
305
|
O—F |
190
|
S—S |
266
|
C ≡ N |
891
|
| C—O |
358
|
O—Cl |
203
|
C = N |
615
|
||
| C—F |
485
|
O—I |
234
|
Si—Si |
340
|
||
| C—Cl |
339
|
Si—H |
393
|
||||
| C—Br |
276
|
F—F |
154
|
Si—C |
360
|
||
| C—I |
240
|
F—Cl |
253
|
Si—O |
452
|
||
| C—S |
259
|
F—Br |
237
|
||||
| Cl—Cl |
239
|
||||||
| Cl—Br |
218
|
||||||
| Br—Br |
193
|
||||||
|
*C = O in (CO2) = 799
|
|||||||
When a bond is strong, there is a higher bond energy because it takes more energy to break a strong bond. This correlates with bond order and bond length. When the Bond order is higher, bond length is shorter, and the shorter the bond length means a greater the Bond Energy because of increased electric attraction. In general, the shorter the bond length, the greater the bond energy.
Bond Breakage and Formation
When a chemical reaction occurs, the atoms in the reactants rearrange their chemical bonds to make products. The new arrangement of bonds does not have the same total energy as the bonds in the reactants. Therefore, when chemical reactions occur, there will always be an accompanying energy change. The energy change of a reaction or the heat of the reaction is called enthalpy. It is represented by the mathematical symbol, ΔH, and is calculated as the difference of the energy required to break the bonds of the reactants minus the energy released by the formation of the bonds in the products.
ΔH = Energy of Reactants – Energy of Products
.jpg?revision=1)
.jpg?revision=1)
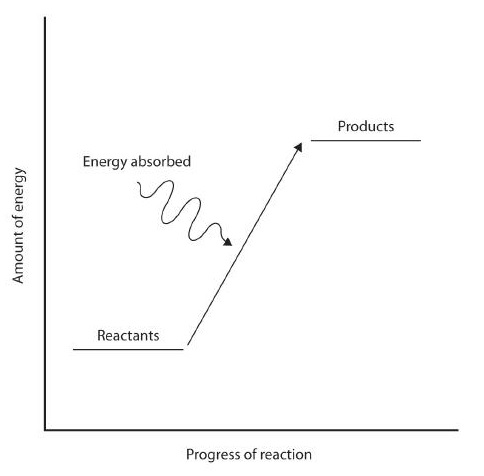
Figure 7.12: Reaction Enthalpy. (upper reaction) Exothermic Reactions. For an exothermic chemical reaction, energy is given off as reactants are converted to products. (lower reaction) Endothermic Reactions. For an endothermic chemical reaction, energy is absorbed as reactants are converted to products.
In some reactions, the energy of the resulting products is lower than the energy of the reactants. Thus, in the course of the reaction, the excess energy released by product formation will be released to the surrounding environment. Such reactions are exothermic and can be represented by an energy-level diagram in Figure 7.12 (upper). In most cases, the energy is given off as heat (although a few reactions give off energy as light). In chemical reactions where the products have a higher energy than the reactants, the reactants must absorb energy from their environment to be able to react. These reactions are endothermic and can be represented by an energy-level diagrams like Figure 7.12 (lower).
Exothermic and endothermic reactions can be thought of as having energy as either a “product” of the reaction or a “reactant”, respectively. Exothermic reactions release energy, so energy is a product. Endothermic reactions require energy, so energy is a reactant.
Exothermic reactions will have a negative overall enthalpy and endothermic reactions will have a positive overall enthalpy.
ΔH = Energy of Reactants – Energy of Products
The energy of a reaction can be treated stoichiometrically within the reaction just like any of the compounds within the reaction. For the combustion of methane, CH4, each mole that is burned will release 824 kJ of energy. Thus, it is easy to calculate how much energy is released for any amount of CH4 that is burned. We can also easily calculate how much CO2 is produced for each mole of CH4 that is burned, since one mole of CO2 is produced for each mole of CH4 burned.
(Back to the Top)
Complete Combustion
Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide (CO2) and water (H2O). It is quite important that you can write properly balanced equations for these reactions, because they often come up as a part of thermochemistry calculations. Some are easier than others. For example, with alkanes, the ones with an even number of carbon atoms are marginally harder than those with an odd number!
Example 1: Propane Combustion
For example, with propane (C3H8), you can balance the carbons and hydrogens as you write the equation down. Your first draft would be:
Counting the oxygens leads directly to the final version:
Example 2: Butane Combustion
With butane (C4H10), you can again balance the carbons and hydrogens as you write the equation down.
Counting the oxygens leads to a slight problem – with 13 on the right-hand side. Having an odd number of oxygens on the product side makes it impossible to balance with the even number on the reactant side. In cases like this, start trying to balance the equation, by changing the cofactor in front of the alkane to 2. Then rebalance the carbon and hydrogens on the product side. In this case, the oxygen number should come out to a positive number and you should now be able to balance the equation.
The hydrocarbons become harder to ignite as the molecules get bigger. This is because the bigger molecules don’t vaporize so easily – the reaction is much better if the oxygen and the hydrocarbon are well mixed as gases. If the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Bigger molecules have greater Van der Waals attractions which makes it more difficult for them to break away from their neighbors and turn to a gas.
Provided the combustion is complete, all the hydrocarbons will burn with a blue flame. However, combustion tends to be less complete as the number of carbon atoms in the molecules rises. That means that the bigger the hydrocarbon, the more likely you are to get a yellow, smoky flame.
Incomplete combustion
Incomplete combustion (where there is not enough oxygen present) can lead to the formation of carbon or carbon monoxide. As a simple way of thinking about it, the hydrogen in the hydrocarbon gets the first chance at the oxygen to form water in the product, and the carbon gets whatever is left over! When only carbon is formed, the presence of glowing carbon particles in a flame turns it yellow, and black carbon is often visible in the smoke. If some oxygen can interact with the carbon, but not enough to form carbon dioxide (CO2), then carbon monoxide (CO) is produced as a colorless poisonous gas.
Why carbon monoxide is poisonous: Oxygen is carried around the blood by hemoglobin, a protein found in red blood cells. Carbon dioxide also binds with hemoglobin. Hemoglobin carries oxygen from your lungs to every cell in your body, where it drops off the oxygen and picks up carbon dioxide and carries it back to the lungs. Hemoglobin will then exchange the carbon dioxide for the oxygen in the air that you breath in. When you exhale, you release the carbon dioxide. Carbon monoxide can also bind to hemoglobin. The difference is that carbon monoxide binds irreversibly (or very strongly) – making that particular molecule of hemoglobin useless for carrying oxygen. If you breath in enough carbon monoxide you will die from an internal form of suffocation!
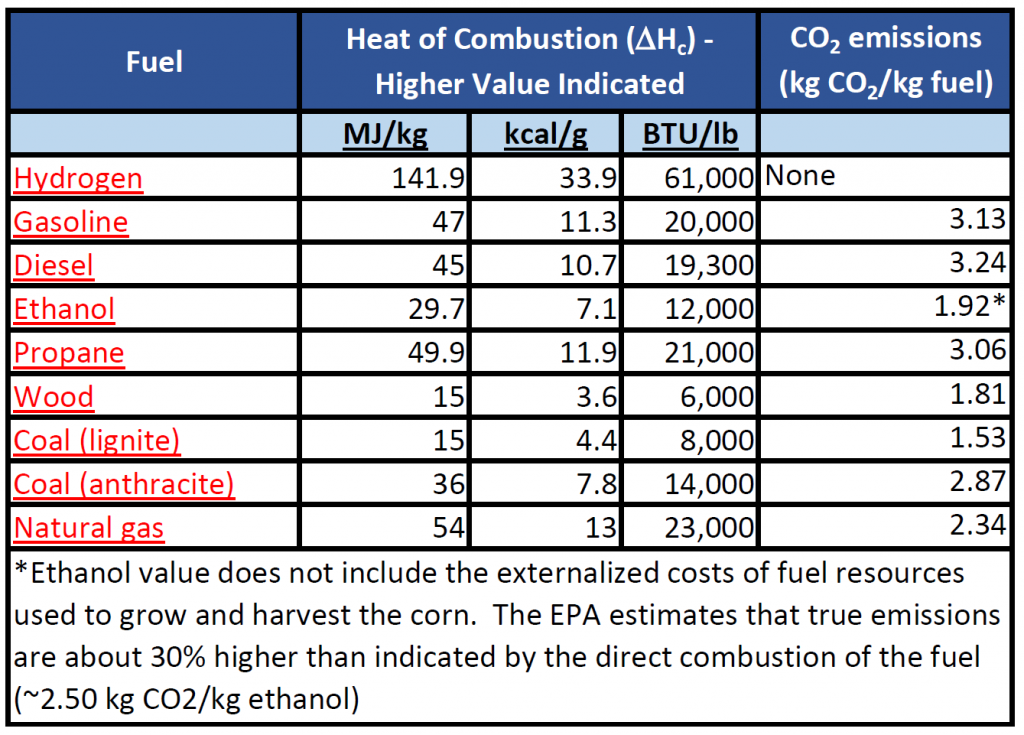
Heat of Combustion
The heat of combustion, ΔHc , is defined as the total energy released as heat when a substance undergoes complete combustion with oxygen under a set of standard conditions. It is calculated the same way that enthalpy is calculated, however, it is specific for a set of standard conditions. Heat of Combustion is a useful value because it is a constant value for the type of material being burned and can be used to compare the efficiency and utility of different fuel sources used most often in society (oil, gasoline, natural gas, diesel, coal, wood, hydrogen, ethanol, etc). In the United States, heating ratings are commonly given in British Thermal Units (BTU) per pound of material. A common conversion to the metric units is:
BTU/lb = (kJ/kg)*2.326
Heat of combustion, ΔHc, values common fuel sources and their associated CO2 emissions are indicated in Table 7.5
Table 7.5 Heat of Combustion and CO2 Emissions Profile of Common Fuels
Adapted from: EPA -(2014) and Wikipedia – Heat of Combustion
Petroleum (from Greek: petra: “rock” + oleum: “oil”) is a naturally occurring, yellow-to-black liquid found in geological formations beneath the Earth’s surface, which is commonly refined into various types of fuels. It consists mainly of alkanes, cycloalkanes, and alkenes of various lengths and some additional minor organic compounds. Alkanes with five or more carbons are liquids and are found as common components of petroleum (also called crude oil). Many cycloalkanes are also found in petroleum products as well including, gasoline, kerosene, diesel, motor oil and many other heavy oils. Natural gas, on the other hand, is composed predominantly of methane (CH4), but also contains ethane (C2H6), propane (C3H8), and butane (C4H10). Natural gas also contains trace levels of nitrogen, carbon dioxide (CO2), and hydrogen sulfide (H2S).
Components of petroleum are separated by size using a technique called fractional distillation. The resulting samples include gasoline with alkanes ranging from five to ten carbons in length and kerosene with a mixture of alkanes ranging in carbon length from ten to seventeen. Alkanes with longer carbon chains are found in diesel fuel, fuel oil, petroleum jelly, paraffin wax, motor oils, and the very highest chain length are used in asphalt. It is estimated that the world uses about 95 million barrels of oil each day! The Environmental Protection Agency estimates that each barrel of oil produces 0.43 metric tons of CO2. Note that a metric ton is equivalent to 1,000 kg. That means that 40,850,000 metric tons of CO2 or 40,850,000,000 kg of CO2 are released into the atmosphere each day just from oil consumption! That is almost 15 billion metric tons of CO2 per year! This calculation only includes the consumption of crude oil and not other common fuel sources, such as natural gas, coal, wood, and renewable energy sources like ethanol and biodiesel.
Up For Discussion:
In Oregon, it is estimated that during the winter months that it take about 40 BTU per hour per square foot to heat an average, well-insulated home. If you owned a 2,000 square foot home, how many BTUs would you require to heat your own for 1 month? (assume 31 days in the month). If you had the choice of heating your house with natural gas, coal (lignite), or wood, how many kg of each of these fuels would be required to heat your home for one month? How much CO2 would be produced by each fuel each winter month?
(Back to the Top)
Halogenation Reactions (Substitution Type)
In the presence of heat or light, alkanes can react with halogens to form alkyl halides (or haloalkanes). This type of reaction is called a substitution reaction, because the halogen atom is taking the place of (or substituting for) one of the hydrogen atoms on the alkane structure. It should be noted that not all of the halogens react in the same way with alkanes.
- The reaction between alkanes and fluorine: This reaction is explosive even in the cold and dark, and you tend to get carbon and hydrogen fluoride produced, rather than the desired substitution reaction. It is of no particular interest to organic chemists, as the reaction can be very dangerous, and it does not yield the desired product. For example, the desired product would be the alkyl fluoride:
But the reaction goes so fast, that this is the result:
- The reaction between alkanes and iodine: Iodine does not react with the alkanes to any extent – at least, under normal lab conditions. So this reaction is not useful either.
- The reactions between alkanes and chlorine or bromine: There is no reaction in the dark, but if light and heat are present, the reaction will produce the desired alkyl halides. Thus, we will focus our discussion on halogenation reactions with chlorine and bromine.
The Reaction of Methane and Chlorine
In the presence of a flame, the reactions are rather like the fluorine one – producing a mixture of carbon and the hydrogen halide. The violence of the reaction drops considerably as you go from fluorine to chlorine to bromine. The interesting reactions happen in the presence of ultra-violet light (sunlight will do). These are photochemical reactions that happen at room temperature. We’ll look at the reactions with chlorine, although the reactions with bromine are similar, but evolve more slowly.
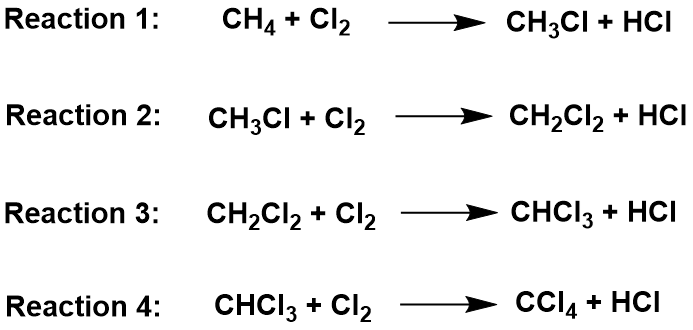
In the substitution reaction, a hydrogen atom in the methane is replaced by a chlorine atom. This can happen multiple times, until all the hydrogens are replaced. Ultimately, the longer the reaction proceeds, the more hydrogens in the alkane are replaced. Thus, you end up with a mixture of chloromethane (CH3Cl), dichloromethane (CH2Cl2), trichloromethane (CHCl3) and tetrachloromethane (CCl4).
The original mixture of a colorless gas (CH4) and a green gas (Cl2) would produce steamy fumes of hydrogen chloride (HCl) and a mist of organic liquids (mixture of the chlorinated methane). All of the organic products are liquid at room temperature with the exception of the chloromethane (CH3Cl) which is a gas.
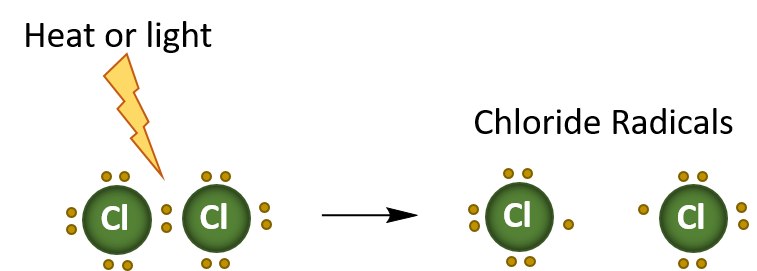
This substitution reaction is an example of a radical reaction, where only one electron is transferred at a time. The heat or light, initiates the reaction by breaking the bond between the two Cl atoms in the chloride ion. This forms two radicals. A radical is an atom, molecule, or ion that has unpaired valence electrons. Thus, they are very unstable and reactive. In the diagram below, the first step of the halogenation reaction is shown below. This is termed initiation.
Initiation Reaction
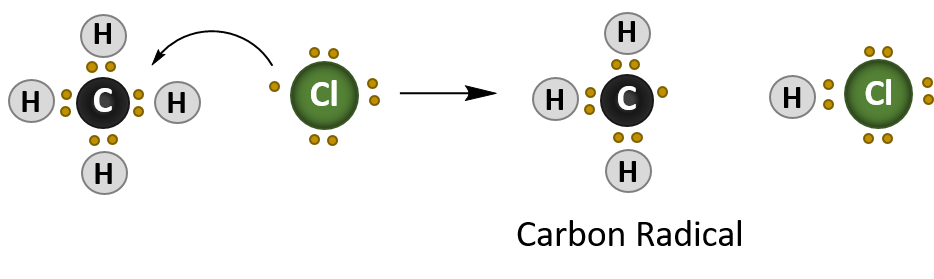
Once the radical is initiated, it will attack the alkane, in this case methane (CH4), and create a new carbon radical. This stage of the reaction is termed propagation, as one radical species creates, or propagates, another radical.
Propagation Reaction
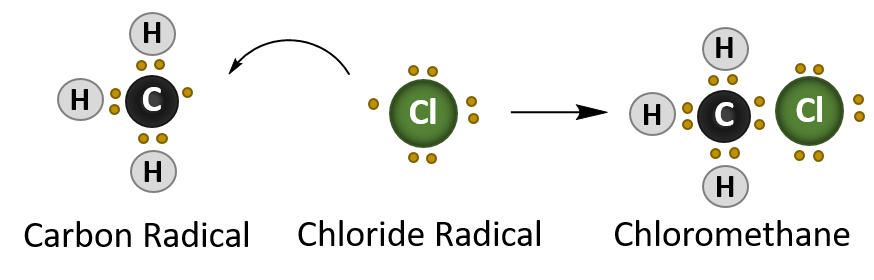
The final stage of a radical reaction is the termination reaction which quenches the radical species present. For the methane – chlorine reaction this is the formation of the chloromethane (CH3Cl).
Termination Reaction
In summary, radical reactions occur in three stages:
- Initiation – where a radical species is generated, generally by heat, light, or other catalytic process.
- Propagation – where one radical species interacts with another molecule to create another radical species.
- Termination – where two radical species interact and quench the radical reaction an form a stable product.
(Back to the Top)
Larger Alkanes and Chlorine
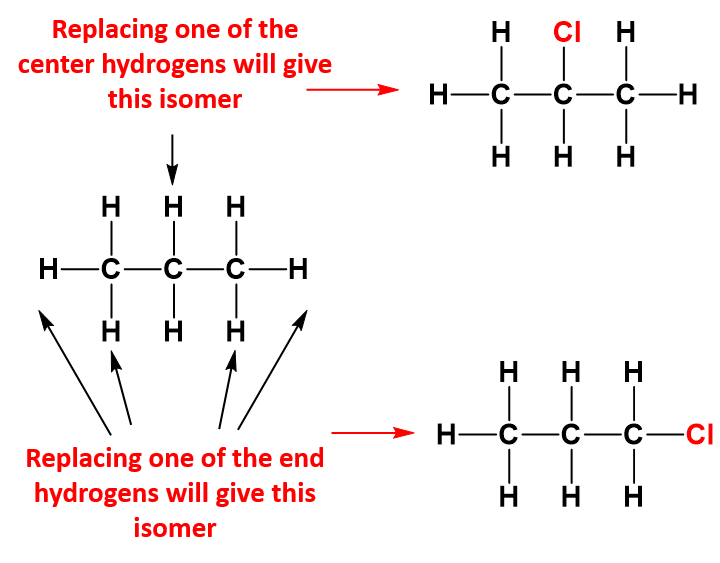
As seen with the methane example, if you halogenate larger alkanes, you would again get a mixture of substitution products, but it is worth just looking briefly at what happens if only one of the hydrogen atoms gets substituted (monosubstitution) – just to show that things aren’t always as straightforward as they seem! For example, with propane, you could get one of two isomers:
If chance was the only factor, you would expect to get three times as much of the isomer with the chlorine on the end. There are 6 hydrogens that could get replaced on the end carbon atoms compared with only 2 in the middle. In fact, you get about the same amount of each of the two isomers. If you use bromine instead of chlorine, the great majority of the product is where the bromine is attached to the center carbon atom. Why does this happen?
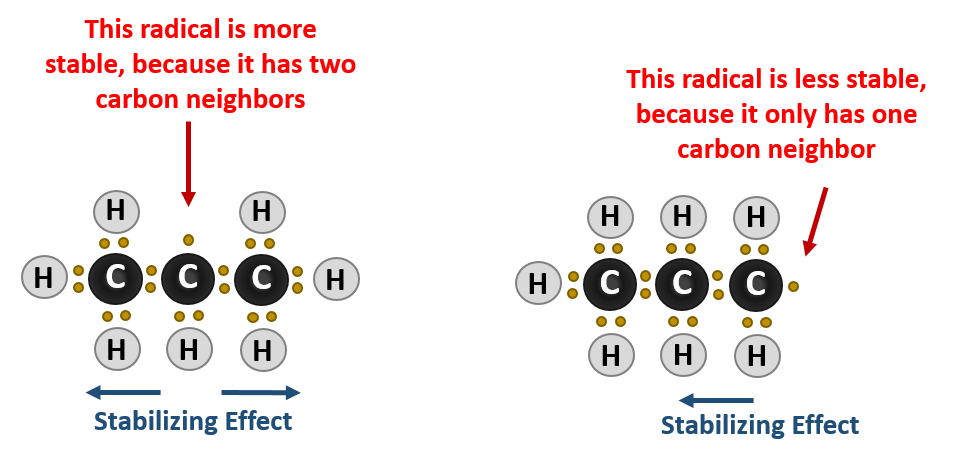
It has to do with the stability of the carbon radical intermediate that forms during the reaction. Carbons that have more carbon neighbors will more easily lose a hydrogen and form a carbon radical intermediate. The neighboring carbons, being larger than the neighboring hydrogen atoms, can help stabilize the formation of the carbon radical. Thus, in the halogenation reaction tertiary carbons will be the most reactive positions, followed by secondary carbons and finally primary carbons. Quaternary carbons are unreactive as they do not have any hydrogens available that can be substituted by a halogen.
Halogenation Reactions with Cycloalkanes
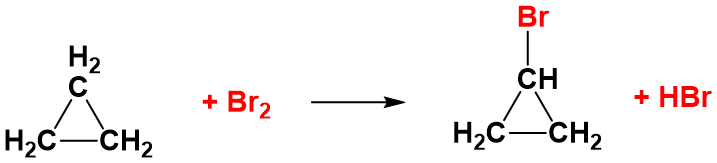
The reactions of the cycloalkanes are generally just the same as the alkanes, with hydrogen atoms on the cyclic ring structure being replaced by the halogen atom. For example in the presence of UV light, cyclopropane will undergo substitution reactions with chlorine or bromine just like a non-cyclic alkane.
However, the small ring structures – particularly cyclopropane – also have the ability to react in the dark. In the absence of UV light, cyclopropane can undergo addition reactions in which the ring is broken. For example, with bromine, cyclopropane gives following linear compound.
This can still happen in the presence of UV light – but you will get a mixture of the substitution reactions as well. The ring is broken because cyclopropane suffers badly from ring strain. Recall that the bond angles in the ring are 60° rather than the normal value of about 109.5° when the carbon makes four single bonds.
Concept Review Exercises
-
Why are alkanes sometimes called paraffins?
-
Which halogen reacts most readily with alkanes? Which reacts least readily?
Answers
-
Alkanes do not react with many common chemicals. They are sometimes called paraffins, from the Latin parum affinis, meaning “little affinity.”
-
most readily: F2; least readily: I2
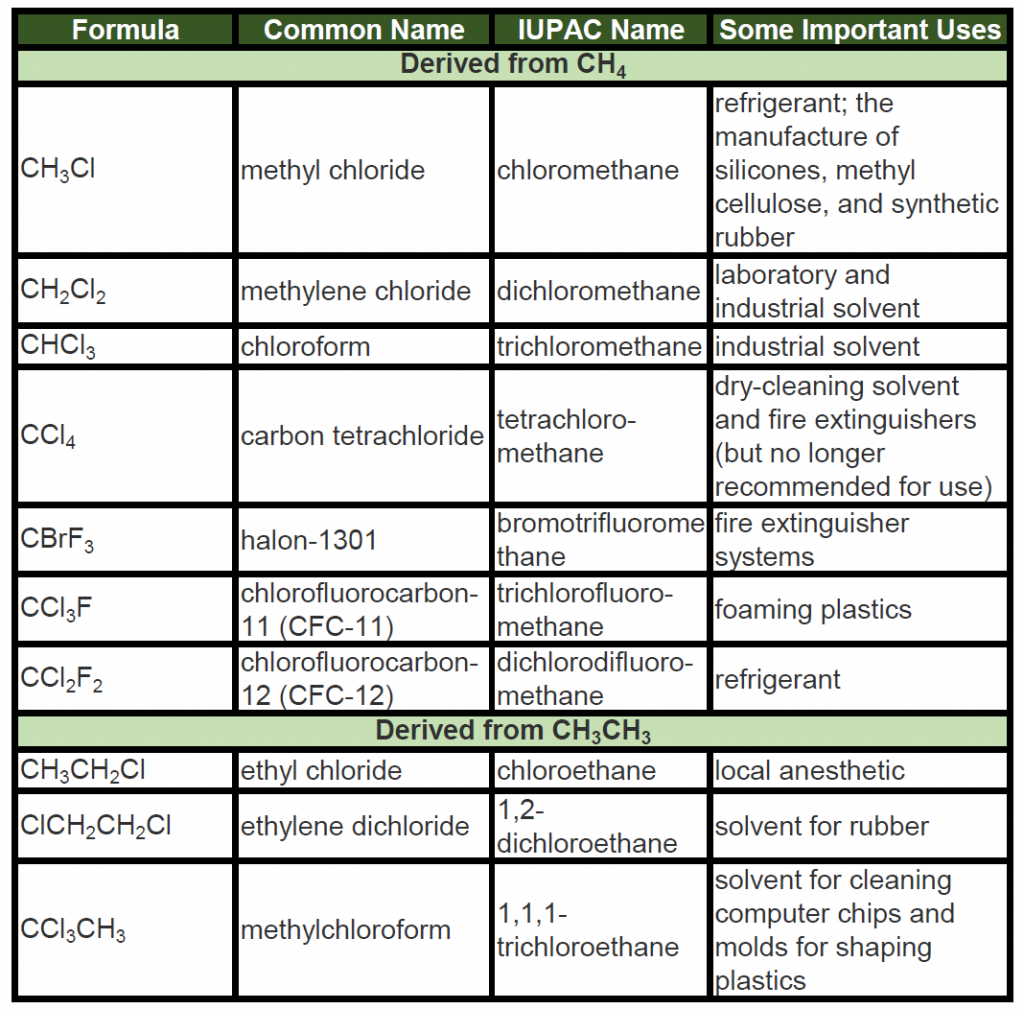
A wide variety of interesting and often useful compounds have one or more halogen atoms per molecule. For example, methane (CH4) can react with chlorine (Cl2), replacing one, two, three, or all four hydrogen atoms with Cl atoms. Several halogenated products derived from methane and ethane (CH3CH3) are listed in Table 7.6 along with some of their uses.
Table 7.6: Some Halogenated Hydrocarbons
To Your Health: Halogenated Hydrocarbons
Once widely used in consumer products, many chlorinated hydrocarbons are suspected carcinogens (cancer-causing substances) and also are known to cause severe liver damage. An example is carbon tetrachloride (CCl4), once used as a dry-cleaning solvent and in fire extinguishers but no longer recommended for either use. Even in small amounts, its vapor can cause serious illness if exposure is prolonged. Moreover, it reacts with water at high temperatures to form deadly phosgene (COCl2) gas, which makes the use of CCl4 in fire extinguishers particularly dangerous.
Ethyl chloride, in contrast, is used as an external local anesthetic. When sprayed on the skin, it evaporates quickly, cooling the area enough to make it insensitive to pain. It can also be used as an emergency general anesthetic.
Bromine-containing compounds are widely used in fire extinguishers and as fire retardants on clothing and other materials. Because they too are toxic and have adverse effects on the environment, scientists are engaged in designing safer substitutes for them, as for many other halogenated compounds.
To Your Health: Chlorofluorocarbons and the Ozone Layer
Alkanes substituted with both fluorine (F) and chlorine (Cl) atoms have been used as the dispersing gases in aerosol cans, as foaming agents for plastics, and as refrigerants. Two of the best known of these chlorofluorocarbons (CFCs) are listed in Table 7.6.
Chlorofluorocarbons contribute to the greenhouse effect in the lower atmosphere. They also diffuse into the stratosphere, where they are broken down by ultraviolet (UV) radiation to release Cl atoms. These in turn break down the ozone (O3) molecules that protect Earth from harmful UV radiation. Worldwide action has reduced the use of CFCs and related compounds. The CFCs and other Cl- or bromine (Br)-containing ozone-destroying compounds are being replaced with more benign substances. Hydrofluorocarbons (HFCs), such as CH2FCF3, which have no Cl or Br to form radicals, are one alternative. Another is hydrochlorofluorocarbons (HCFCs), such as CHCl2CF3. HCFC molecules break down more readily in the troposphere, and fewer ozone-destroying molecules reach the stratosphere.
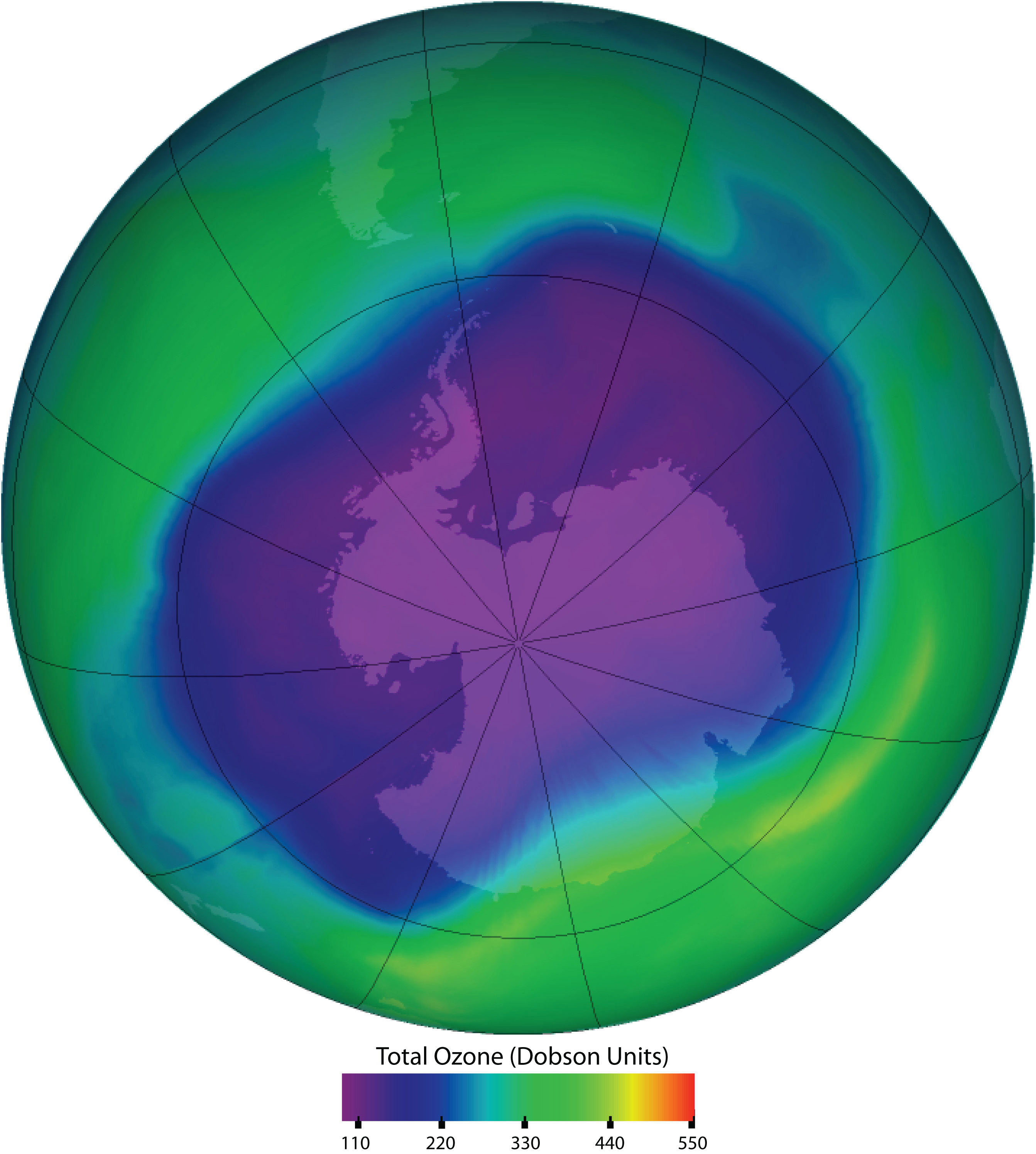
Figure 7.13. Ozone Depletion in the Upper Atmosphere. Ozone in the upper atmosphere shields Earth’s surface from UV radiation from the sun, which can cause skin cancer in humans and is also harmful to other animals and to some plants. Ozone “holes” in the upper atmosphere (the gray, pink, and purple areas at the center) are large areas of substantial ozone depletion. They occur mainly over Antarctica from late August through early October and fill in about mid-November. Ozone depletion has also been noted over the Arctic regions. The largest ozone hole ever observed occurred on 24 September 2006.
Source: Image courtesy of NASA.
(Back to the Top)
Cracking Alkanes
What is cracking? Cracking is the name given to breaking up large hydrocarbon molecules into smaller and more useful bits. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst. A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
The source of the large hydrocarbon molecules is often the naphtha fraction or the gas oil fraction from the fractional distillation of crude oil (petroleum). These fractions are obtained from the distillation process as liquids, but are re-vaporized into the gaseous phase before cracking. There isn’t any single unique reaction happening in the cracker. The hydrocarbon molecules are broken up in a fairly random way to produce mixtures of smaller hydrocarbons, some of which have carbon-carbon double bonds. One possible reaction involving the hydrocarbon C15H32 might be:
Or, showing more clearly what happens to the various atoms and bonds:
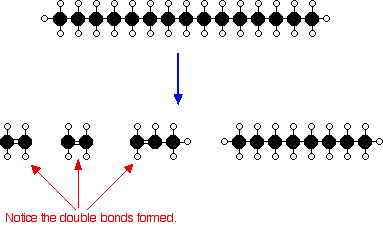
This is only one way in which this particular molecule might break up. Notice that in addition to producing smaller alkanes, the cracking reaction can also produce alkenes with double bonds. In this case, the alkenes – ethene (C2H4) and propene (C3H6) – are important materials for making plastics or producing other organic chemicals. The octane is one of the molecules found in petrol (gasoline).
Looking Closer: An Alkane Basis for Properties of Other Compounds
An understanding of the physical properties of the alkanes is important in that petroleum and natural gas and the many products derived from them—gasoline, bottled gas, solvents, plastics, and more—are composed primarily of alkanes. This understanding is also vital because it is the basis for describing the properties of other organic and biological compound families. For example, large portions of the structures of lipids consist of nonpolar alkyl groups. Lipids include the dietary fats and fatlike compounds called phospholipids and sphingolipids that serve as structural components of living tissues. These compounds have both polar and nonpolar groups, enabling them to bridge the gap between water-soluble and water-insoluble phases. This characteristic is essential for the selective permeability of cell membranes.
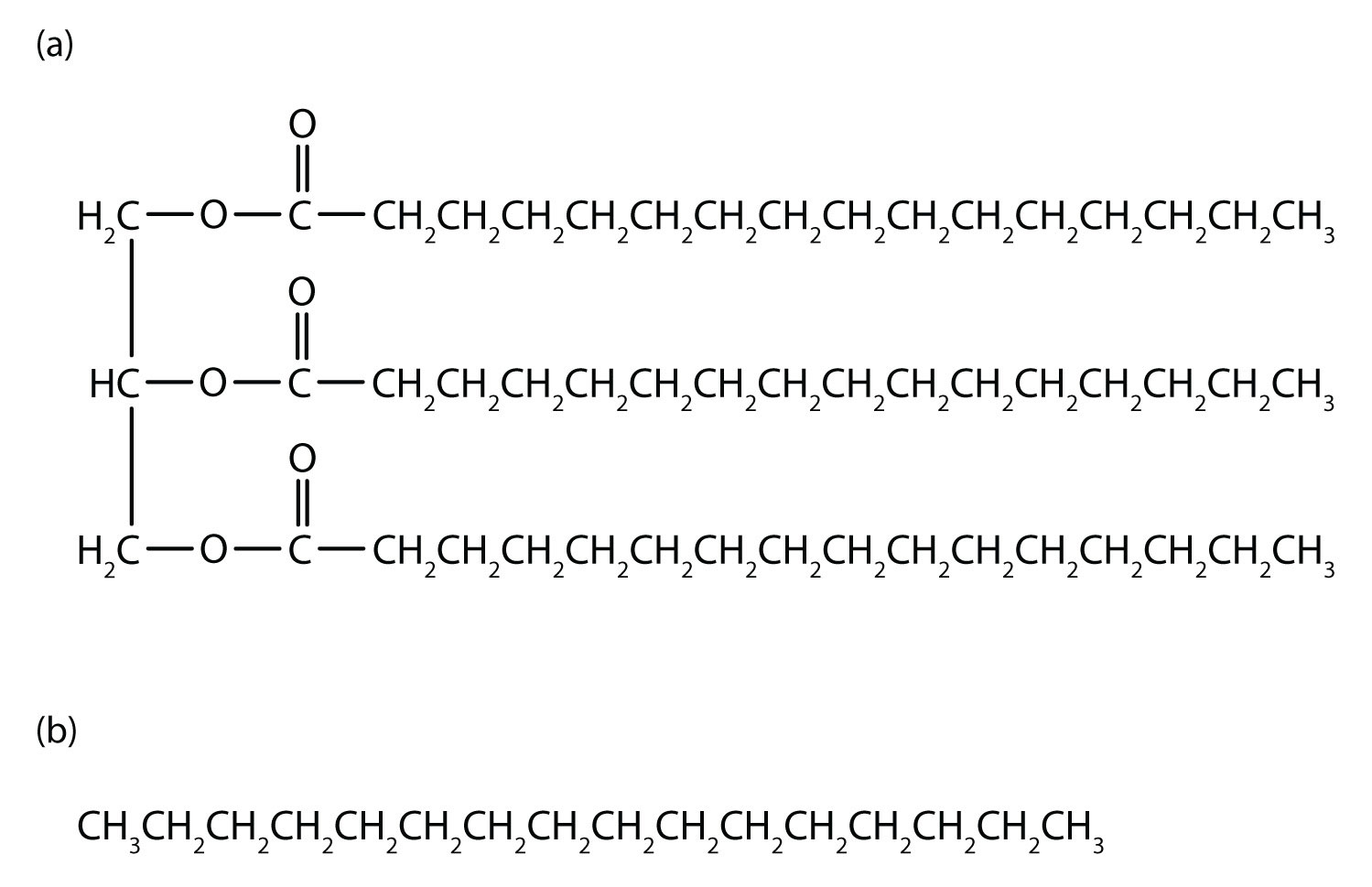
Figure 7.13 Comparision of Lipids and Alkanes. Tripalmitin (a), a typical fat molecule, has long hydrocarbon chains typical of most lipids. Compare these chains to hexadecane (b), an alkane with 16 carbon atoms.
(Back to the Top)
7.5 Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the summary and ask yourself how they relate to the topics in the chapter.
Organic chemistry is the chemistry of carbon compounds, and inorganic chemistry is the chemistry of all the other elements. Carbon atoms can form stable covalent bonds with other carbon atoms and with atoms of other elements, and this property allows the formation the tens of millions of organic compounds. Hydrocarbons contain only hydrogen and carbon atoms.
Hydrocarbons in which each carbon atom is bonded to four other atoms are called alkanes or saturated hydrocarbons. They have the general formula CnH2n + 2. Any given alkane differs from the next one in a series by a CH2 unit. Any family of compounds in which adjacent members differ from each other by a definite factor is called a homologous series.
Carbon atoms in alkanes can form straight chains or branched chains. Two or more compounds having the same molecular formula but different structural formulas are isomers of each other. There are no isomeric forms for the three smallest alkanes; beginning with C4H10, all other alkanes have isomeric forms.
Cycloalkanes are hydrocarbons whose molecules are closed rings rather than straight or branched chains. A cyclic hydrocarbon is a hydrocarbon with a ring of carbon atoms.
Recall that a structural formula shows all the carbon and hydrogen atoms and how they are attached to one another. A condensed structural formula shows the hydrogen atoms right next to the carbon atoms to which they are attached. A line-angle formula is a formula in which carbon atoms are implied at the corners and ends of lines. Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds
The physical properties of alkanes reflect the fact that alkane molecules are nonpolar. Alkanes are insoluble in water and less dense than water.
Alkanes are generally unreactive toward laboratory acids, bases, oxidizing agents, and reducing agents. They do burn (undergo combustion reactions).
Alkanes react with halogens by substituting one or more halogen atoms for hydrogen atoms to form halogenated hydrocarbons. This reaction is called halogenation. An alkyl halide (haloalkane) is a compound resulting from the replacement of a hydrogen atom of an alkane with a halogen atom.
Larger alkanes can be broken into smaller alkanes and alkenes using high heat or catalysts. These reactions are called cracking reactions.
(Back to the Top)
7.6 End-of-Chapter Exercises
-
You find an unlabeled jar containing a solid that melts at 48°C. It ignites readily and burns readily. The substance is insoluble in water and floats on the surface. Is the substance likely to be organic or inorganic?
-
What is the danger in swallowing a liquid alkane?
- Distinguish between lighter and heavier liquid alkanes in terms of their effects on the skin.
-
Following is the line formula for an alkane. Draw the condensed structure.

-
Write equations for the complete combustion of each compound.
- butane, C4H10 (a common lighter fluid)
- octane, C8H18 (a typical hydrocarbon in gasoline).
-
The density of a gasoline sample is 0.690 g/mL. On the basis of the complete combustion of octane, calculate the amount in grams of carbon dioxide (CO2) and water (H2O) formed per gallon (3.78 L) of the gasoline when used in an automobile.
-
Draw the structures for four of the nine isomeric hexanes (C7H16).
-
Indicate whether the structures in each set represent the same compound or isomers.
-
-
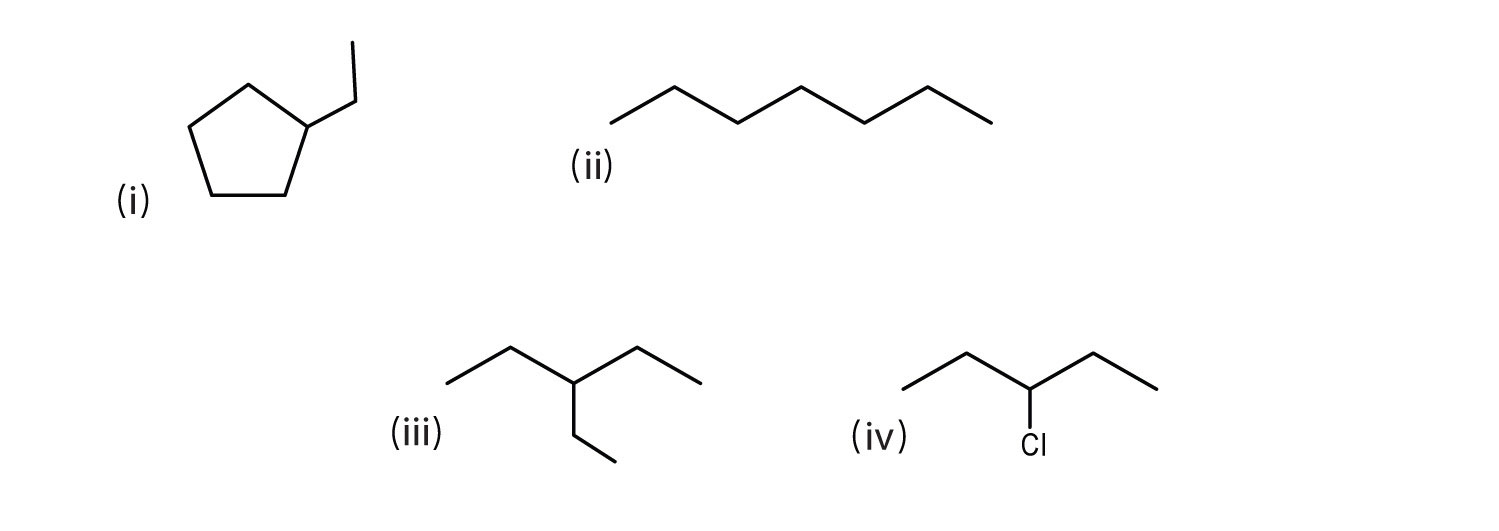
Consider the line-angle formulas shown here and answer the questions.

- Which pair of formulas represents isomers? Draw each structure.
- Which formula represents an alkyl halide? Write its condensed structural formula.
- Which formula represents a cyclic alkane?
- That is the molecular formula of the compound represented by (i)?
(Back to the Top)
7.7 References
Text for this chapter has been adapted from the creative commons resources listed below, unless otherwise noted in the text.
- Organic Chemistry (2016) Libretexts, U.C. Davis, Licenced under: Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License. Available at: https://chem.libretexts.org/Core/Organic_Chemistry
- Inhalant. (2017, February 12). In Wikipedia, The Free Encyclopedia. Retrieved 01:21, February 13, 2017, from https://en.wikipedia.org/w/index.php?title=Inhalant&oldid=765135147
- Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
- Physical and Theoretical Chemistry (2017) Libretexts, U.C. Davis, Licenced under: Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Bond_Energies.
- Petroleum. (2017, February 14). In Wikipedia, The Free Encyclopedia. Retrieved 06:29, February 16, 2017, from https://en.wikipedia.org/w/index.php?title=Petroleum&oldid=765440823
- Ball, D.W., Hill, J.W., and Scott, R.J. (2016) MAP: The Basics of General, Organic and Biological Chemistry. Libre Texts. Available at: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry_Textbook_Maps/Map%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)