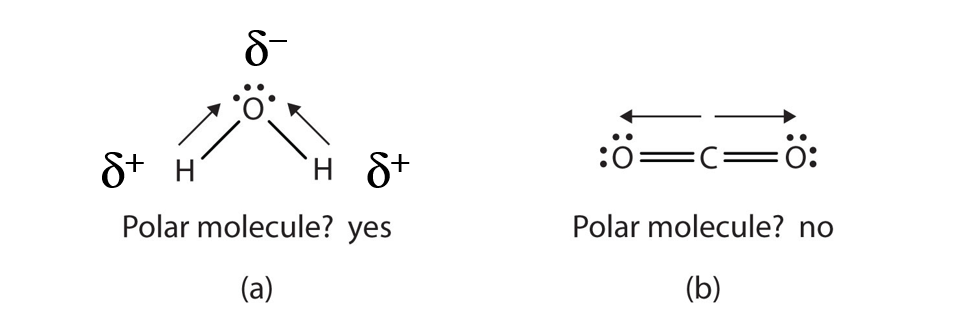Home » Student Resources » Online Chemistry Textbooks » CH105: Consumer Chemistry » CH105: Chapter 4 – The Shape and Characteristics of Compounds
MenuCH105: Consumer Chemistry
Chapter 4 – The Shape and Characteristics of Compounds
This content can also be downloaded as a Interactive PDF File. For the interactive PDF, adobe reader is required for full functionality.
This text is published under creative commons licensing, for referencing and adaptation, please click here.
Sections:
4.1 Molar Mass
4.2 Electronegativity and Bond Polarity
4.3 Shape of Ionic Compounds
4.4 Shape of Covalent Compounds: VSEPR Theory
Predicting the Shape: The AXE Method
4.5 Intermolecular Interactions
Chapter Summary
References
4.1 Molar Mass
The molar mass of an ionic or covalent compound is simply the sum of the masses of its atoms. To calculate a molar mass, it is important that you keep track of the number of atoms of each element in the chemical formula to obtain the correct molecular mass.
For Example:
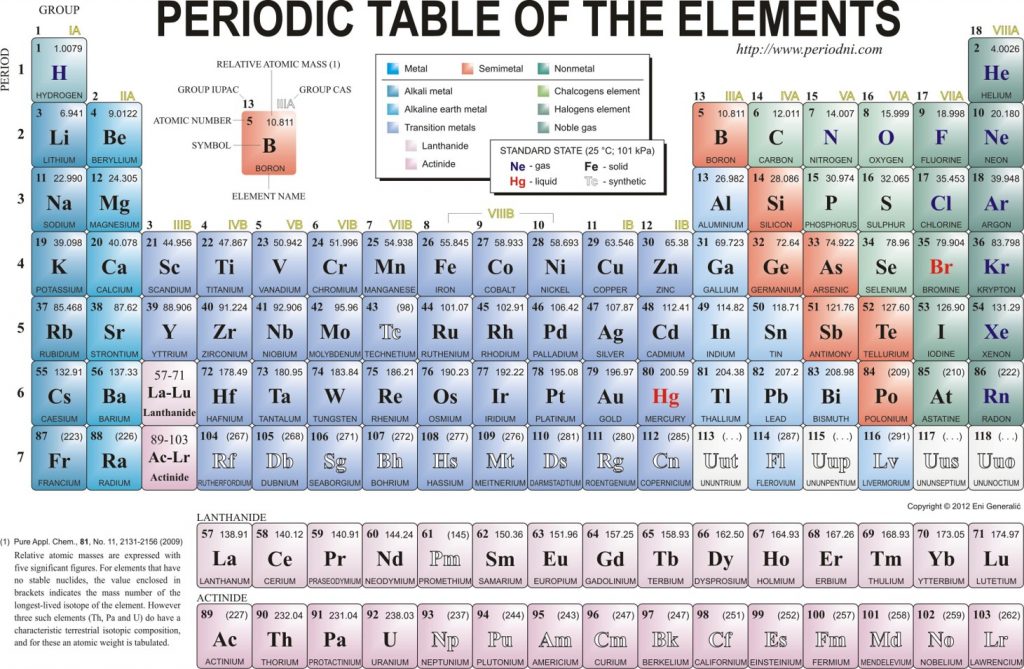
A molecule of NaCl contains 1 Na+ and 1 Cl-. Thus, we can calculate the molar mass of this compound by adding together the atomic masses of sodium and chlorine, as found on the periodic table (Figure 4.1).
Figure 4.1 Periodic Table of the Elements
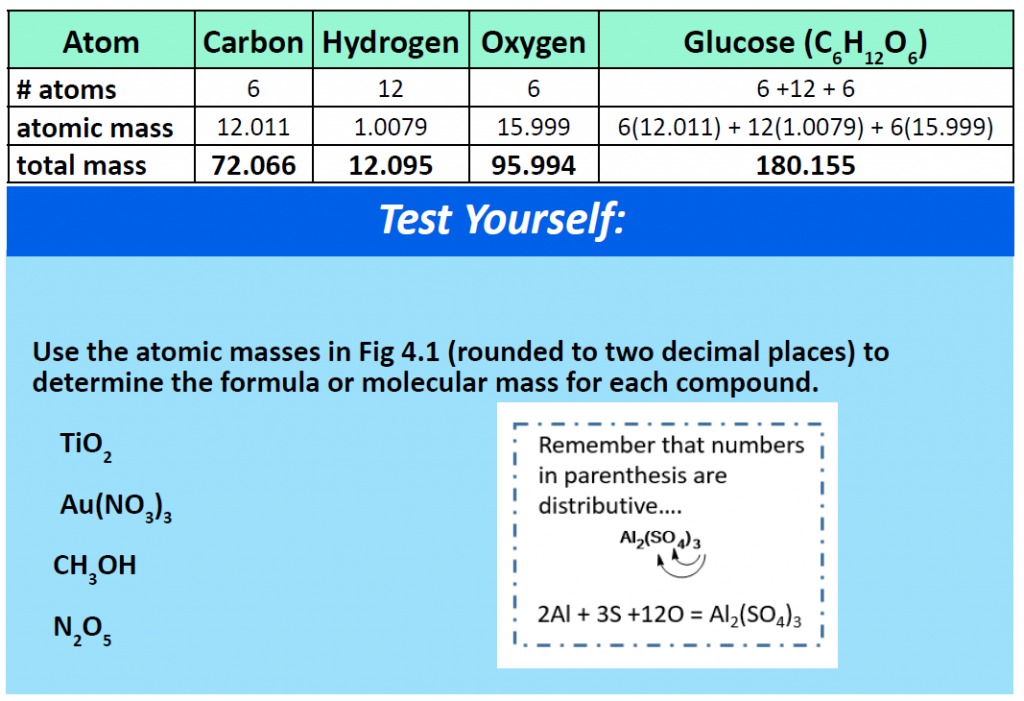
For a larger molecule, like glucose (C6H12O6) that has multiple atoms of the same type, simply multiply the atomic mass of each atom by the number of atoms present in the chemical formula, and then add up all the atomic masses to get the final molecular mass.
4.2 Electronegativity and Bond Polarity
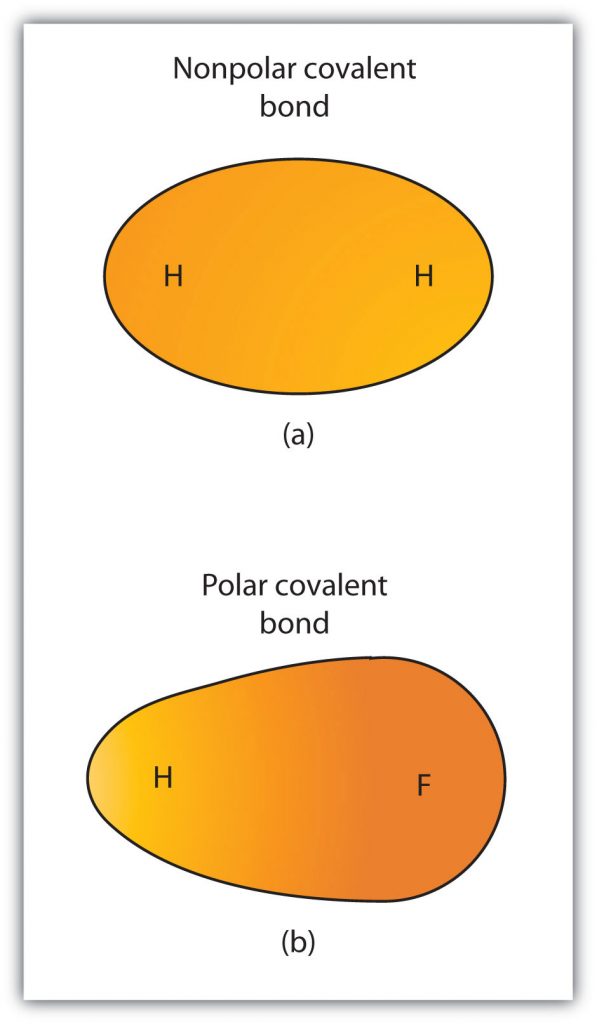
Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. Unless the bond connects two atoms of the same element, there will always be one atom that attracts the electrons in the bond more strongly than the other atom does, as shown in Figure 4.2. When such an imbalance occurs, there is a resulting buildup of some negative charge (called a partial negative charge and designated δ−) on one side of the bond and some positive charge (designated δ+) on the other side of the bond. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure 4.2, is called a polar covalent bond. A covalent bond that has an equal sharing of electrons (part (a) of Figure 4.2) is called a nonpolar covalent bond.
Figure 4.2 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. This is a nonpolar covalent bond. (b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. This is a polar covalent bond.
Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred completely rather than shared. To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond.
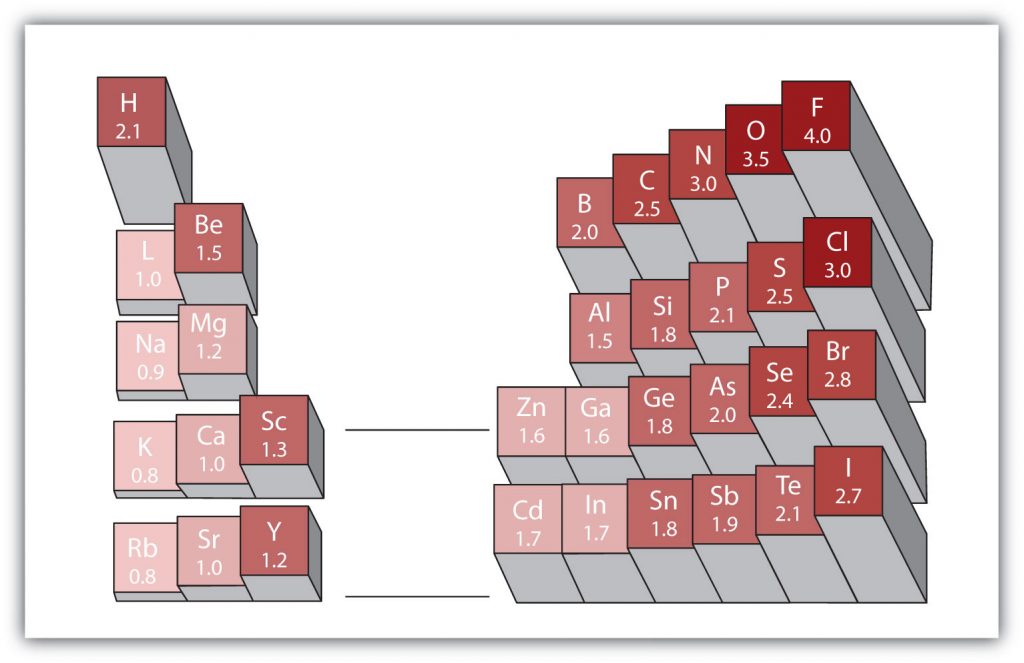
There are various numerical scales for rating electronegativity. Figure 4.3 shows one of the most popular—the Pauling scale. The polarity of a covalent bond can be judged by determining the difference in the electronegativities between the two atoms making the bond. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond.
Figure 4.3 Electronegativities of Various Elements. The Pauling Scale for electronegativities has the value for fluorine atoms set at 4.0, the highest value.
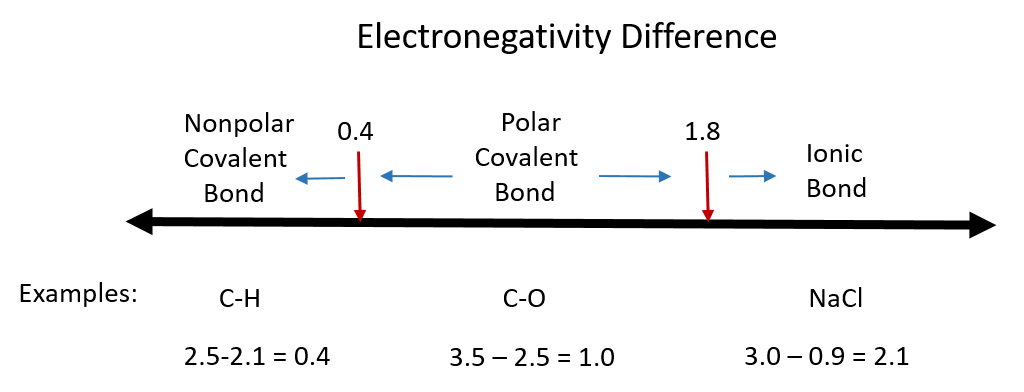
Although there are no hard and fast rules, the general rule is that a difference in electronegativity less than 0.4 indicates the bond is nonpolar; when the difference is greater than 0.4, the bond is considered polar. When the difference in electronegativities is large enough (generally greater than about 1.8), the resulting compound is considered ionic rather than covalent. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond. Examples of electronegativity difference are shown in Figure 4.4.
Figure 4.4 Electronegativity Difference Diagram. The diagram above is a guide for discerning what type of bond forms between two different atoms. By taking the difference between the electronegativity values for each of the atoms involved in the bond, the bond type and polarity can be predicted.
When a molecule’s bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. For example, the orientation of the two O–H bonds in a water molecule (Figure 4.5) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties. (For example, the boiling point of water [100°C] is high for such a small molecule and is due to the fact that polar molecules attract each other strongly.) In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other in the molecule and so cancel each other’s effects. Thus, carbon dioxide molecules are nonpolar overall. This lack of polarity influences some of carbon dioxide’s properties. (For example, carbon dioxide becomes a gas at −77°C, almost 200° lower than the temperature at which water boils.)
Figure 4.5 Physical Properties and Polarity. The physical properties of water and carbon dioxide are affected by their molecular polarities. Note that the arrows in the diagram always point in the direction where the electrons are more strongly attracted.
4.3 Shape of Ionic Compounds
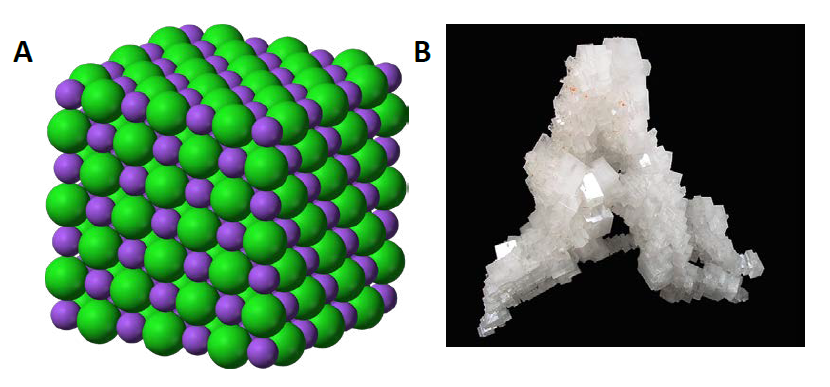
In chemistry, an ionic compound is a chemical compound comprised ions held together by electrostatic forces, termed ionic bonding. The compound is neutral overall, but consists of positively charged cations and negatively charged anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH4+) and carbonate (CO32-) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbours, so are not considered to be part of molecules, but instead part of a continuous three-dimensional network, usually in a crystalline structure. Figure 4.6 shows the structure of sodium chloride (NaCl)
Figure 4.6 Crystal Lattice. (A) The crystal structure of sodium chloride, NaCl, a typical ionic compound. The purple spheres represent sodium cations, Na+, and the green spheres represent chloride anions, Cl−. (B) Halite, the mineral form of sodium chloride, forms when salty water evaportates leaving the ions behind.
Source: (A) Benjah-bmm27 (2010). (B) Lavisky, R. (2010) Both (A) and (B) Available at: https://en.wikipedia.org/wiki/Ionic_compound
Ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing hydroxide (OH−) or oxide (O2−) ions are classified as bases. All other ionic compounds without these ions are known as salts. Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids, they are most often electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
FOR A COOL VIDEO ON THE OXIDATIVE PROPERTIES OF SALTPETER, CLICK HERE!
4.4 Shape of Covalent Compounds: VSEPR Theory
Unlike ionic compounds, with their extended crystal lattices, covalent molecules are discrete units with specific three-dimensional shapes. The shape of a molecule is determined by the fact that covalent bonds, which are composed of shared negatively charged electrons, tend to repel one another. This concept is called the valence shell electron pair repulsion (VSEPR) theory.
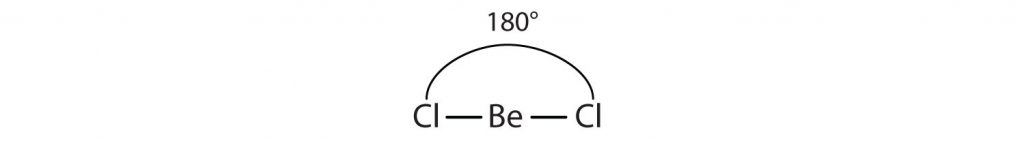
For example, the two covalent bonds in BeCl2 stay as far from each other as possible, ending up 180° apart from each other. The result is a linear molecule:
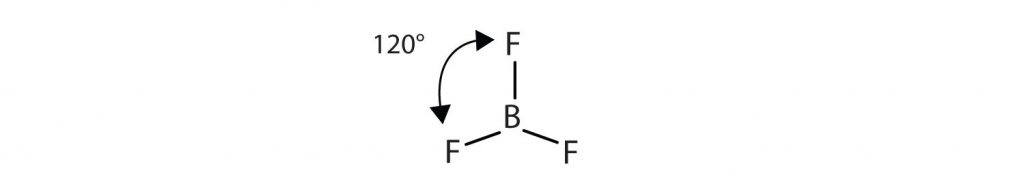
Similarly, the three covalent bonds in BF3 repel each other to form 120° angles in a plane, in a shape called trigonal planar:
The molecules BeCl2 and BF3 actually violate the octet rule; however, such exceptions are rare and will not be discussed in this text.
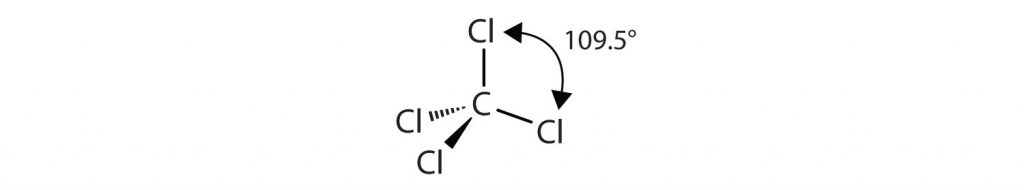
The four covalent bonds in CCl4 arrange themselves three dimensionally, pointing toward the corner of a tetrahedron and making bond angles of 109.5°:
Predicting the Shape: The AXE Method
So, how can this theory of electron repulsion be used in a simple way to predict the shape of a molecule? First, it is necessary to understand how many electron pairs are involved and whether or not those electron pairs are in bonded relationships between two atoms (Bonded Pairs) or whether they are Lone Pairs. To make this determination, it is useful to draw the Lewis Structure for the molecule and show all of the bonding groups and lone pair electrons. Note that in VSEPR theory that a double or triple bond are treated as a single bonding group, because all of the electrons involved in the bond are shared with only a single atom. The sum of the number of atoms bonded to a central atom and the number of lone pairs formed by the nonbonding valence electrons is known as the central atom’s steric number. Once the Lewis Structure is drawn and the central atom’s steric number is known, the AXE method can be used to predict the overall shape of the molecule.
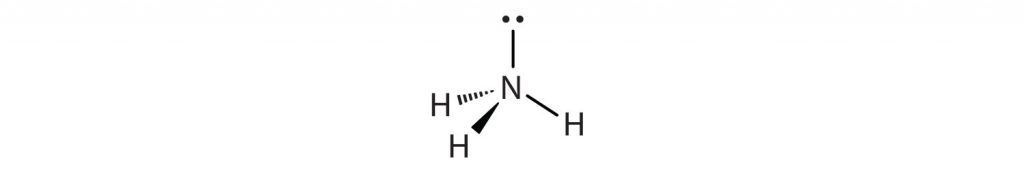
In the AXE method of electron counting the ‘A’ refers to the central atom in the molecule, ‘X’ is the number of bonded atoms connected to the central atom, and ‘E’ are the number of lone pair electrons present on the central atom. Note that ‘X’ and ‘E’ only refer to the bonded atoms and electron pairs associated with the central atom ‘A’. The number of connected atoms, ‘X’, and lone pair electrons, ‘E’ are then written as a formula. For example, if you have a molecule of NH3:
We can see that there are three atoms of hydrogen bonded to the central nitrogen atom.
Thus, ‘X’ = 3 bonded atoms. We can also see that the central nitrogen has one lone pair of electrons extending from the top of the atom. Thus, ‘E’ = 1 lone pair of electrons. We derive two important pieces of information from this. First, we can add ‘X’ + ‘E’ to determine the steric number of our central atom. In this case, the nitrogen has a steric number of 4 = (3 + 1). Second, we can solve our overall AXE formula by writing in the subscripts for ‘X’ and ‘E’. For NH3, the AXE formula is AX3E1. With the steric number and AXE formula calculated, we can now use Table 4.1 to predict the molecular geometry or shape of the overall molecule.
Table 4.1: AXE Model of Molecular Shapes
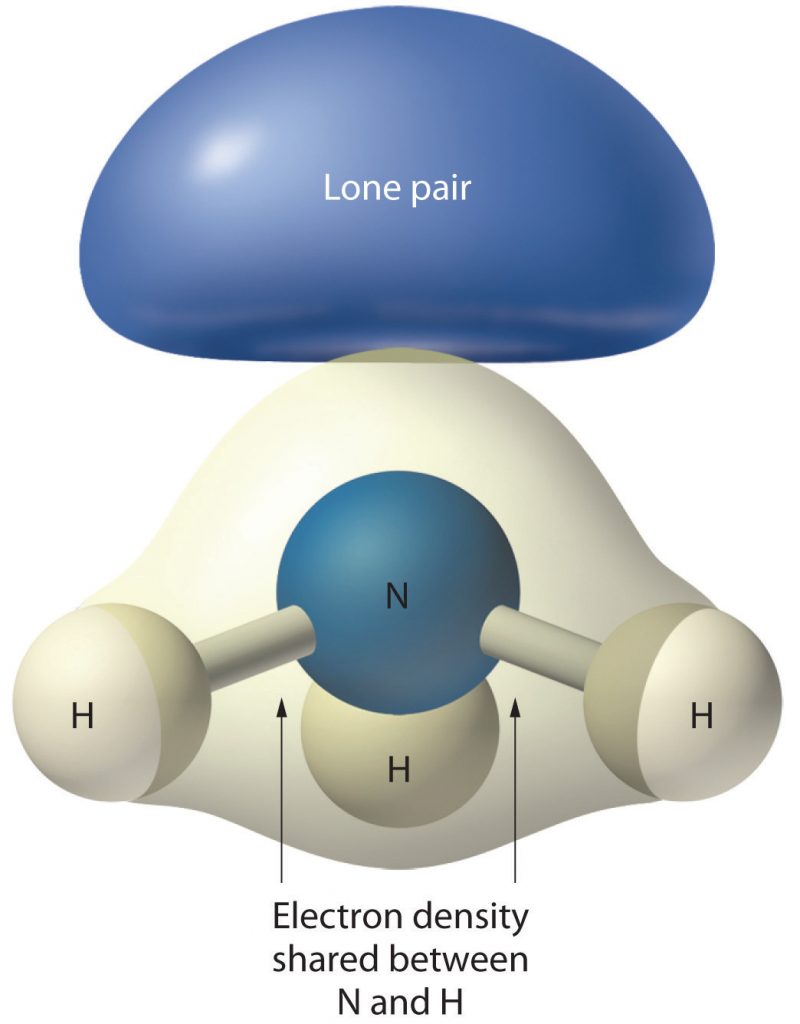
In Table 4.1, scroll down to the correct steric number row, in this case, row 4, and then scan across to find the correct AXE formula for your compound. In this case, the second selection is correct: AX3E1. So we can see from this table that the shape of NH3 is trigonal pyramidal (or it looks like a pyramid with three corners with a hydrogen at each one. Notice that a lone pair electrons on the central atom affect the shape by their presence by pushing the hydrogens below the central plain of the molecule, but that it is not included in the overall shape of the molecule (Figure 4.7).
Figure 4.7 The Molecular Geometry of Ammonia (NH3). The lone pair density in NH3 contributes to the overall shape of the molecule by pushing the hydrogens below the plain of the nitrogen central atom. However, they are not visible in the final molecular geometry, which is trigonal pyramidal.
Source: http://2012books.lardbucket.org/books/principles-of-general-chemistry-v1.0/index.html
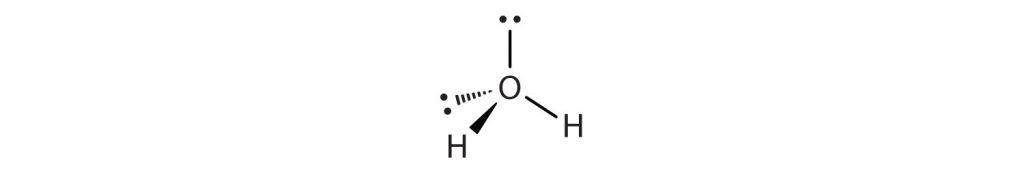
In a water molecule, oxygen has 2 Lone Pairs of electrons and 2 bonded hydrogen atoms, giving it a steric number of 4 and an AXE formula of AX2E2. Using Table 4.1, we see that the shape of H2O is bent.
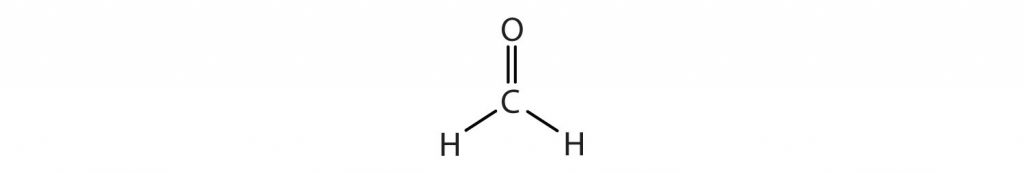
How about the shapes of molecules with multiple bonds? They are determined by treating the multiple bonds as one bonding group. Thus, CH2O has a shape similar to that of BF3:
This is because CH2O has three bonded atoms, or an ‘X’ = 3, and carbon has no lone pairs, so ‘E’ = 0. The steric number is 3, and the AXE formula is AX3E0. Since ‘E’ = 0, we can drop it from the equation to give AX3.
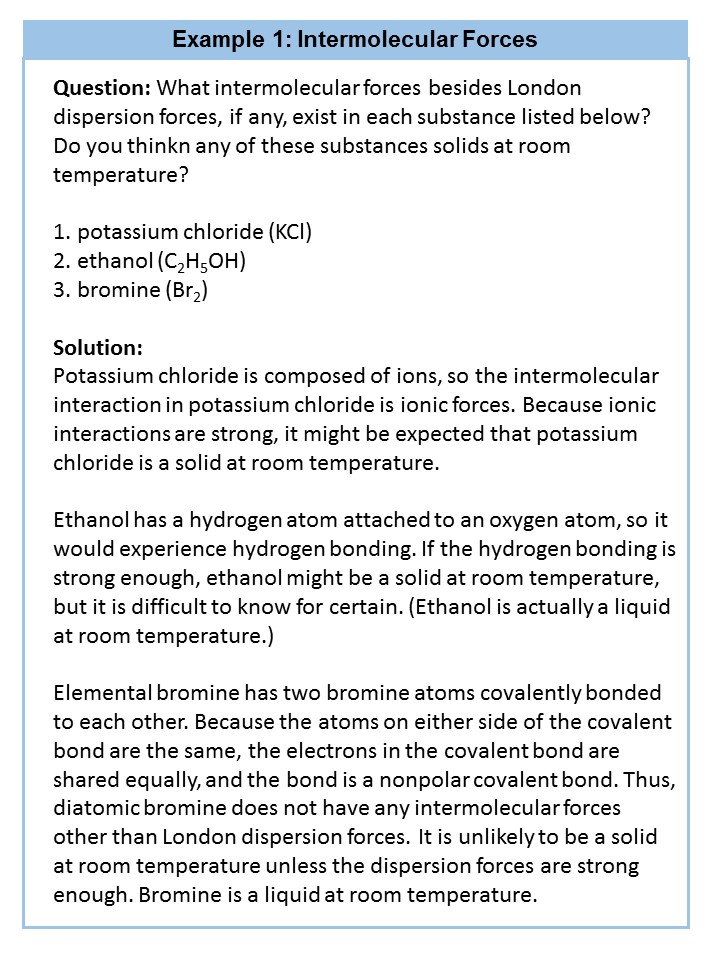
4.5 Intermolecular Interactions
In addition to learning about the bond characteristics and shapes of molecules, it is also very important to learn about how molecules interact with other molecules around them. This type of interaction, known as an intermolecular interaction, is important for determining broader characteristics of the molecule including reactivity and function.
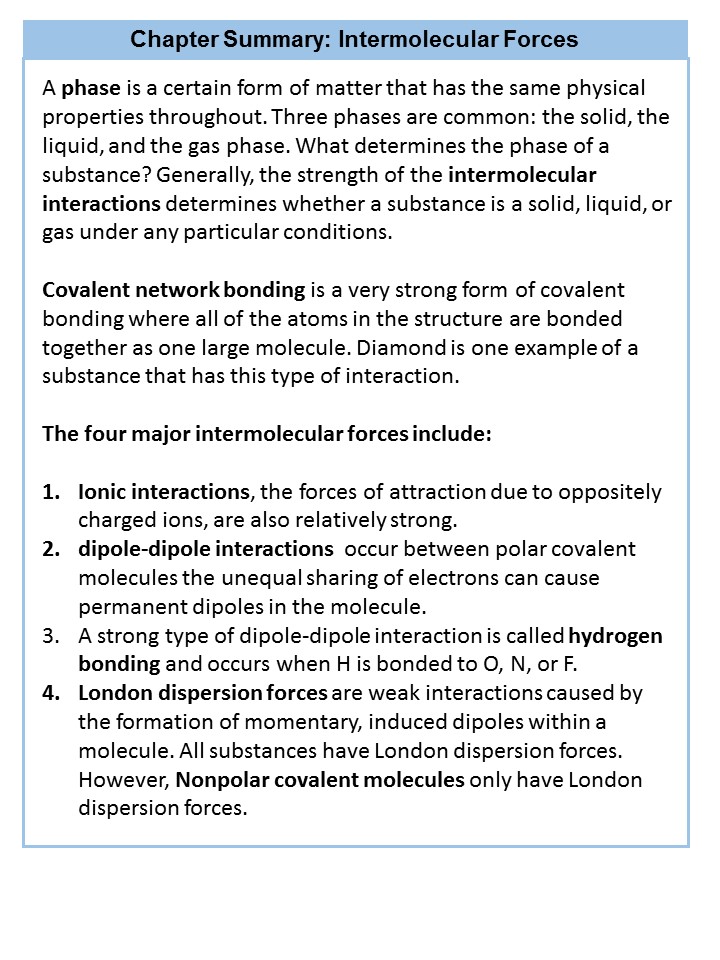
Intermolecular interactions between molecules are dependent on the phase that the molecule exists. A phase is a certain form of matter that includes a specific set of physical properties. That is, the atoms, the molecules, or the ions that make up the phase do so in a consistent manner throughout the phase. As mentioned in Chapter 1, science recognizes three stable phases: the solid phase, in which individual particles can be thought of as in contact and held in place (defined volume and shape); the liquid phase, in which individual particles are in contact but moving with respect to each other (defined volume but, shape of the container); and the gas phase (no defined shape or volume), in which individual particles are separated from each other by relatively large distances. Not all substances will readily exhibit all phases on the Earth. For example, carbon dioxide does not exhibit a liquid or solid phase on Earth unless the pressure is greater than about six times normal atmospheric pressure. Other substances, especially complex organic molecules, may decompose or breakdown at higher temperatures, rather than becoming a liquid or a gas. For example, think about roasting a marshmallow. If it gets too close to the flames it will become charred and blackened, breaking down the sugar molecules inside. The sugar is not converted into the liquid or gaseous phase. Thus, water is very unique in its ability to exist on the Earth in all three phase states (solid ice – liquid water – water vapor).
Which phase a substance adopts depends on the pressure and the temperature it experiences. Of these two conditions, temperature variations are more obviously related to the phase of a substance. When it is very cold, H2O exists in the solid form as ice. When it is warmer, the liquid phase of H2O is present. At even higher temperatures, H2O boils and becomes steam (gaseous phase).
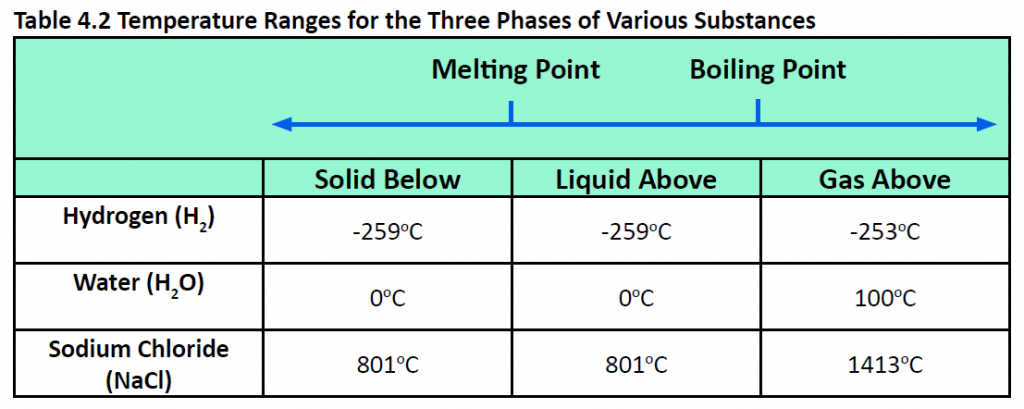
Pressure changes can also affect the presence of a particular phase (as we indicated for carbon dioxide), but its effects are less obvious most of the time. We will mostly focus on the temperature effects on phases, mentioning pressure effects only when they are important. Most chemical substances follow the same pattern of phases when going from a low temperature to a high temperature: the solid phase, then the liquid phase, and then the gas phase. However, the temperatures at which these phases are present differ for all substances and can be rather extreme. Table 4.2 shows the temperature ranges for solid, liquid, and gas phases for three substances. As you can see, there is extreme variability in the temperature ranges. Recall that the melting point of a substance is the temperature that separates a solid and a liquid. The boiling point of a substance is the temperature that separates a liquid and a gas.
What accounts for this variability? Why do some substances become liquids at very low temperatures, while others require very high temperatures before they become liquids? It all depends on the strength of the intermolecular interactions between the particles of substances. (Although ionic compounds are not composed of discrete molecules, we will still use the term intermolecular to include interactions between the ions in such compounds.) Substances that experience strong intermolecular interactions require higher temperatures to become liquids and, finally, gases. Substances that experience weak intermolecular interactions do not need much energy (as measured by temperature) to become liquids and gases and will exhibit these phases at lower temperatures.
Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid. Similarly, solids melt when the molecules acquire enough thermal energy to overcome the intermolecular forces that lock them into place in the solid.
Intermolecular forces are electrostatic in nature; that is, they arise from the interaction between positively and negatively charged species. Like covalent and ionic bonds, intermolecular interactions are the sum of both attractive and repulsive components. Because electrostatic interactions fall off rapidly with increasing distance between molecules, intermolecular interactions are most important for solids and liquids, where the molecules are close together. These interactions become important for gases only at very high pressures, where they are responsible for the observed deviations from the ideal gas law at high pressures.
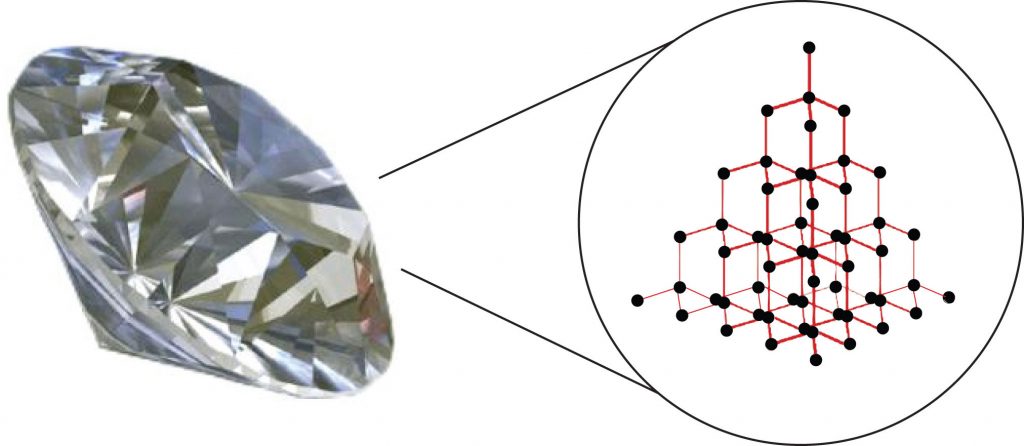
Substances with the highest melting and boiling points have covalent network bonding. This type of interaction is actually a covalent bond. In these substances, all the atoms in a sample are covalently bonded to the other atoms; in effect, the entire sample is essentially one large molecule. Many of these substances are solid over a large temperature range because it takes a lot of energy to disrupt all the covalent bonds at once. One example of a substance that shows covalent network bonding is diamond (Figure 4.8), which is a form of pure carbon. At temperatures over 3,500°C, diamond finally vaporizes into gas-phase atoms.
Figure 4.8. Diamond. Diamond, a form of pure carbon, has covalent network bonding. It takes a very high temperature—over 3,500°C—for diamond to leave the solid state.
Source: Photo © Thinkstock
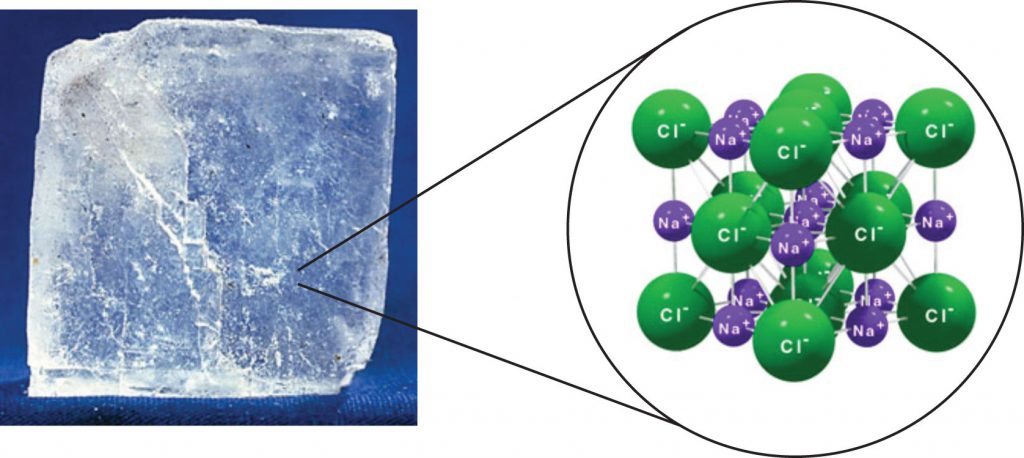
For interactions between different molecules, the strongest force between any two particles is the ionic bond, in which two ions of opposing charge are attracted to each other. Thus, ionic interactions between particles are an intermolecular interaction. Substances that contain ionic interactions are strongly held together, so these substances typically have high melting and boiling points. Sodium chloride (Figure 4.9) is an example of a substance whose particles experience ionic interactions (Table 4.2).
Figure 4.9 Sodium Chloride. Solid NaCl is held together by ionic intermolecular forces. Source: Photo © Thinkstock
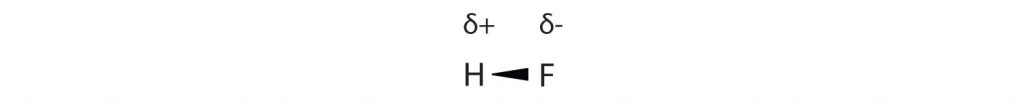
Many substances that experience covalent bonding exist as discrete molecules and do not engage in covalent network bonding. Thus, most covalently bonded molecules will also experience intermolecular forces. These intermolecular forces are weaker than those found in ionic interactions and depend on the polarity of the covalent bond. Recall that in polar covalent bonds, the electrons that are shared in a covalent bond are not shared equally between the two atoms in the bond. Typically, the atom displaying higher electronegativity attracts the electrons more strongly than the other, leading to the unequal sharing of electrons in the bond. This sets up a permanent dipole within the molecule, where one end of the molecule has a partial negative charge (δ−) and one end has a partial positive charge (δ+). This idea is illustrated in Figure 4.10, which shows a diagram of the covalent bond in hydrogen fluoride (HF).
Figure 4.10 Polar Covalent Bonds. The electrons in the HF molecule are not equally shared by the two atoms in the bond. Because the fluorine atom has nine protons in its nucleus, it attracts the negatively charged electrons in the bond more than the hydrogen atom does with its one proton in its nucleus. Thus, electrons are more strongly attracted to the fluorine atom, leading to an imbalance in the electron distribution between the atoms. The fluorine side of the bond picks up a partial overall negative charge (represented by the δ− in the diagram), while the hydrogen side of the bond has an overall partial positive charge (represented by the δ+ in the diagram). Such a bond is called a polar covalent bond.
The fluorine atom attracts the electrons in the bond more than the hydrogen atom does. The result is an unequal distribution of electrons in the bond, favoring the fluorine side of the covalent bond. Because of this unequal distribution, the fluorine side of the covalent bond actually takes on a partial negative charge (indicated by the δ− in Figure 4.10), while the hydrogen side of the bond, being electron deficient, takes on a partial positive charge (indicated by the δ+ in Figure 4.10). A covalent bond that has an unequal sharing of electrons is called a polar covalent bond. (A covalent bond that has an equal sharing of electrons, as in a covalent bond with the same atom on each side, is called a nonpolar covalent bond.) A molecule with a net unequal distribution of electrons in its covalent bonds is a polar molecule. HF is an example of a polar molecule.
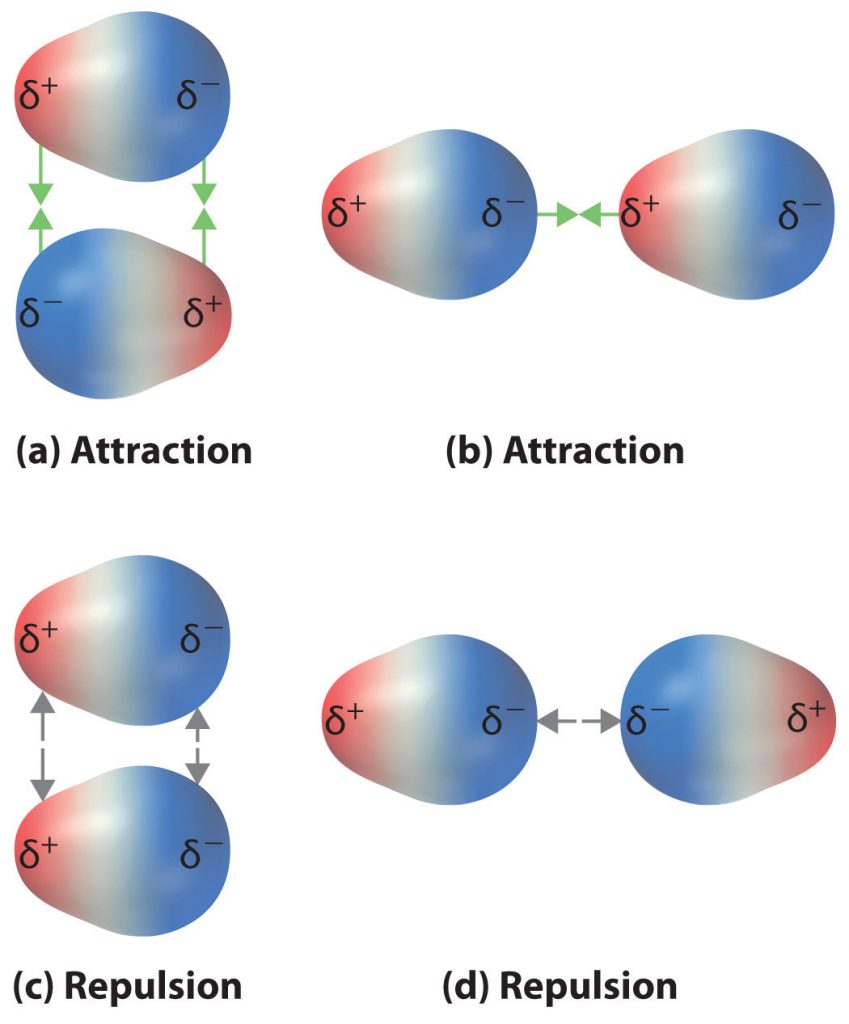
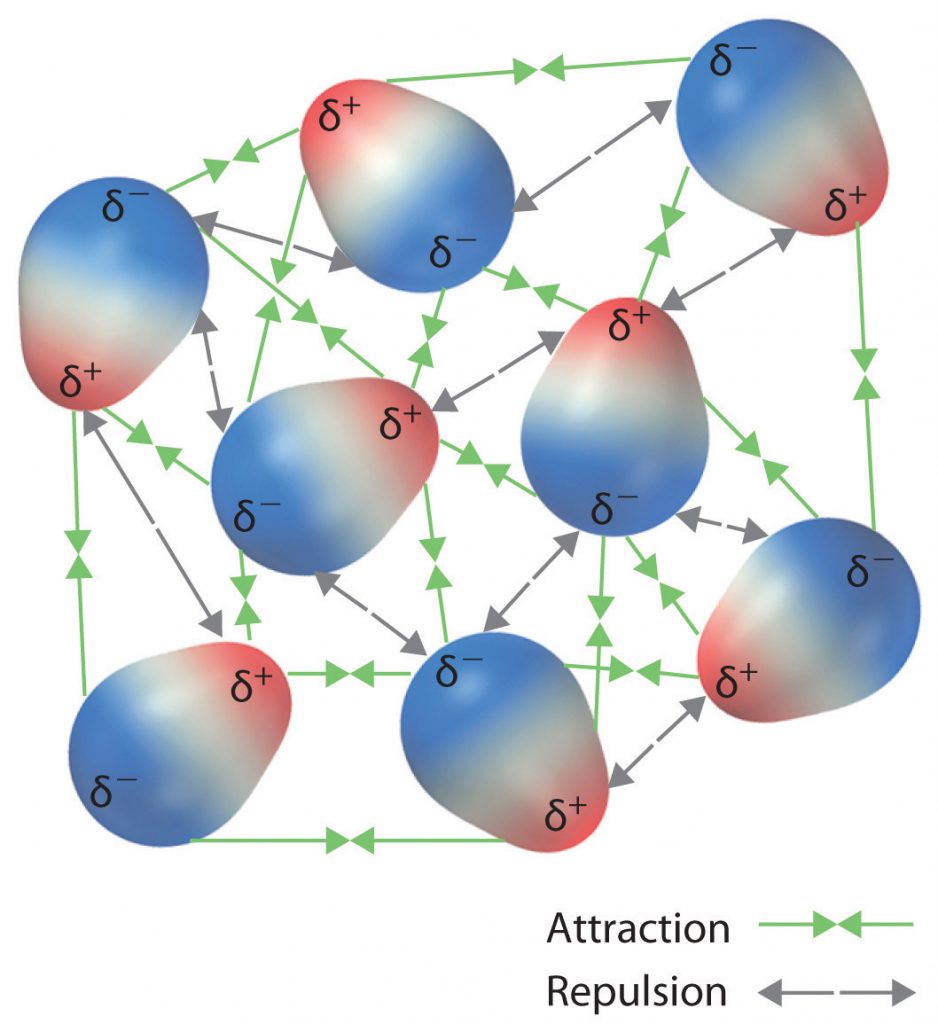
The charge separation in a polar covalent bond is not as extreme as is found in ionic compounds, but there is a related result: oppositely charged ends of different molecules will attract each other. This type of intermolecular interaction is called a dipole-dipole interaction. If the structure of a molecule is polar, then the molecule has a net dipole moment. Molecules with net dipole moments tend to align themselves so that the positive end of one dipole is near the negative end of another and vice versa, as shown in part (a) in Figure 4.11. These arrangements are more stable than arrangements in which two positive or two negative ends are adjacent (Figure 4.11, part c). Hence dipole–dipole interactions, such as those in part (b) in Figure 4.11, are attractive intermolecular interactions, whereas those in part (d) in Figure 4.11 are repulsive intermolecular interactions. Because molecules in a liquid move freely and continuously, molecules always experience both attractive and repulsive dipole–dipole interactions simultaneously, as shown in Figure 4.12. On average, however, the attractive interactions dominate.
Figure 4.11 Attractive and Repulsive Dipole-Dipole Interactions. (a and b) Molecular orientations in which the positive end of one dipole (δ+) is near the negative end of another (δ−) (and vice versa) produce attractive interactions. (c and d) Molecular orientations that juxtapose the positive or negative ends of the dipoles on adjacent molecules produce repulsive interactions.
Figure 4.12 Both Attractive and Repulsive Dipole-Dipole Interactions Occur in a Liquid Sample with Many Molecules.
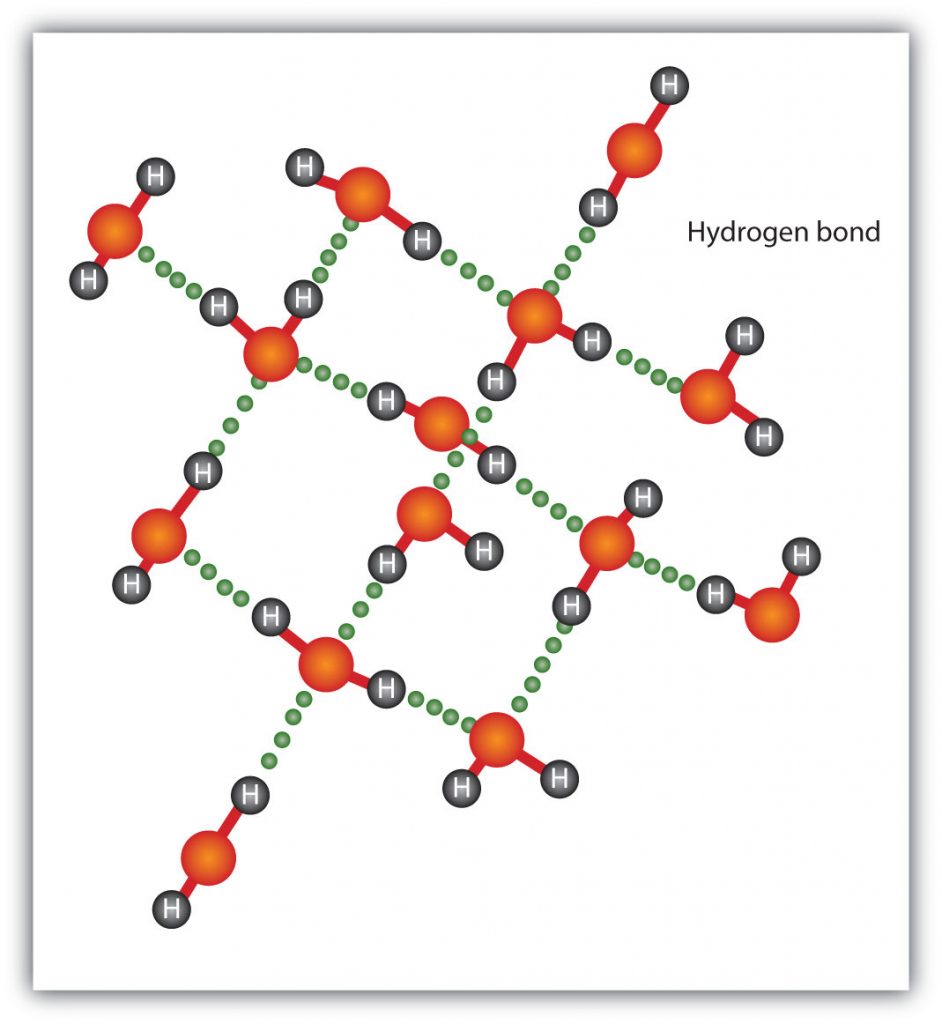
The H–F, O–H, and N–H bonds are strongly polar; In molecules that have these bonds, particularly strong dipole-dipole interactions (as strong as 10% of a true covalent bond) can occur. Because of this strong interaction, hydrogen bonding is used to describe this dipole-dipole interaction. The physical properties of water, which has two O–H bonds, are strongly affected by the presence of hydrogen bonding between water molecules. Figure 4.13 shows how molecules experiencing hydrogen bonding can interact in water.
Figure 4.13 Hydrogen Bonding between Water Molecules. The presence of hydrogen bonding in molecules like water can have a large impact on the physical properties of a substance.
Finally, there are forces between all molecules that are caused by electrons being in different places in a molecule at any one time, which sets up a temporary separation of charge that disappears almost as soon as it appears and sets up a momentary ‘induced dipole’. These are very weak intermolecular interactions and are called London dispersion forces. Since electrons naturally orbit the nucleus of the atom, there are momentary dipoles that are present in the atom as the electrons are shifting from one side to the other. If other atoms are in close proximity, the electrons of the other atoms will orbit in concert with the neighboring atom, i.e. the electrons of one atom are repulsive to the electrons of the neighboring atoms, such that when they are close to the neighboring atom, the neighboring electrons will shift away to the other side of the atom. Thus, the electon movements between atoms of different molecules will synchronize and orbit in a pattern that maximizes the distance between electrons of a neighboring atom. Note that all substances experience London dispersion forces. However, these are the only intermolecular forces that nonpolar covalent compounds experience. Nonpolar covalent molecules tend to be soft in the solid phase and have relatively low melting points. Butter fat would be a good example of a nonpolar covalent compound.
Because London dispersion forces are caused by the instantaneous distribution of electrons in a molecule, larger molecules with a large number of electrons can experience higher levels of London dispersion forces. Examples include waxes, which are long hydrocarbon chains that are solids at room temperature because the molecules have so many electrons. The resulting dispersion forces between these molecules make them assume the solid phase at normal temperatures.
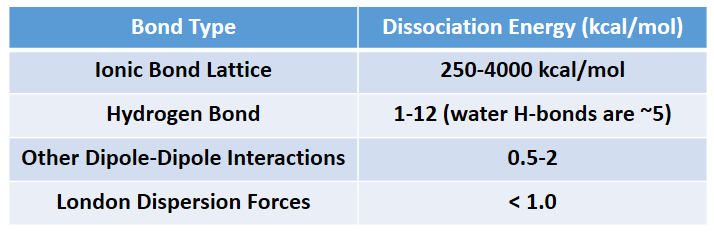
The phase that a substance adopts depends on the type and magnitude of the intermolecular interactions the particles of a substance experience. If the intermolecular interactions are relatively strong, then a large amount of energy—in terms of temperature—is necessary for a substance to change phases. If the intermolecular interactions are weak, a low temperature is all that is necessary to move a substance out of the solid phase. Overall, Ionic interactions are the strongest intermolecular forces followed by hydrogen bonding, other dipole-dipole interactions, and lastly, induced dipoles (London dispersion forces). Intermolecular force strength is indicated in Table 4.3.
Table 4.3 Strength of Intermolecular Forces
Source: https://en.wikipedia.org/wiki/Intermolecular_force
Chapter Summary:
References:
Chapter 4 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
4. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12.
5. Wikipedia, Ionic Compound. Available at: https://en.wikipedia.org/wiki/Ionic_compound