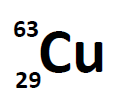Home » Student Resources » Online Chemistry Textbooks » CH105: Consumer Chemistry » Ch105: Chapter 2 – Atoms, Elements and The Periodic Table
MenuCH105: Consumer Chemistry
Chapter 2 – Atoms, Elements, and the Periodic Table
This content can also be downloaded as an printable PDF or an Interactive PDF. For the interactive PDF, adobe reader is required for full functionality.
This text is published under creative commons licensing, for referencing and adaptation, please click here.
Sections:
2.1 What is Organic Chemistry?
2.2 Elements, Atoms, and the Periodic Table
Elements and Abundance
Atomic Theory
Subatomic Particles
Protons Determine the Identity of an Element
Isotopes and Atomic Mass
Electrons and the Periodic Table of the Elements
Features of the Periodic Table
2.3 Chapter Summary
2.4 References
2.1 What is Organic Chemistry?
Have you ever wondered why some plants can be used to make medicines while others are toxic and can kill you? Or why some foods are thought of as healthy while others are bad for you? Or how beverages like beer, cider and wine are made? This course is designed to introduce the reader to fundamental concepts in Organic Chemistry using consumer products, technologies and services as model systems to teach these core concepts and show how organic chemistry is an integrated part of everyday life.
Organic chemistry is a growing subset of chemistry. To put it simply, it is the study of all carbon-based compounds; their structure, properties, and reactions and their use in synthesis. It is the chemistry of life and includes all substances that have been derived from living systems. The application of organic chemistry today can be seen everywhere you look, from the plastic making up components of your computer, to nylon which make up your clothes, to macromolecules and cells that make up your very body! Organic chemistry has expanded our world of knowledge and it is an essential part of the fields of medicine, biochemistry, biology, industry, nanotechnology, rocket science, and many more!
To begin our discussions of organic chemistry, we need to first take a look at chemical elements and understand how they interact to form chemical compounds.
2.2 Elements, Atoms, and the Periodic Table
Elements and Abundance
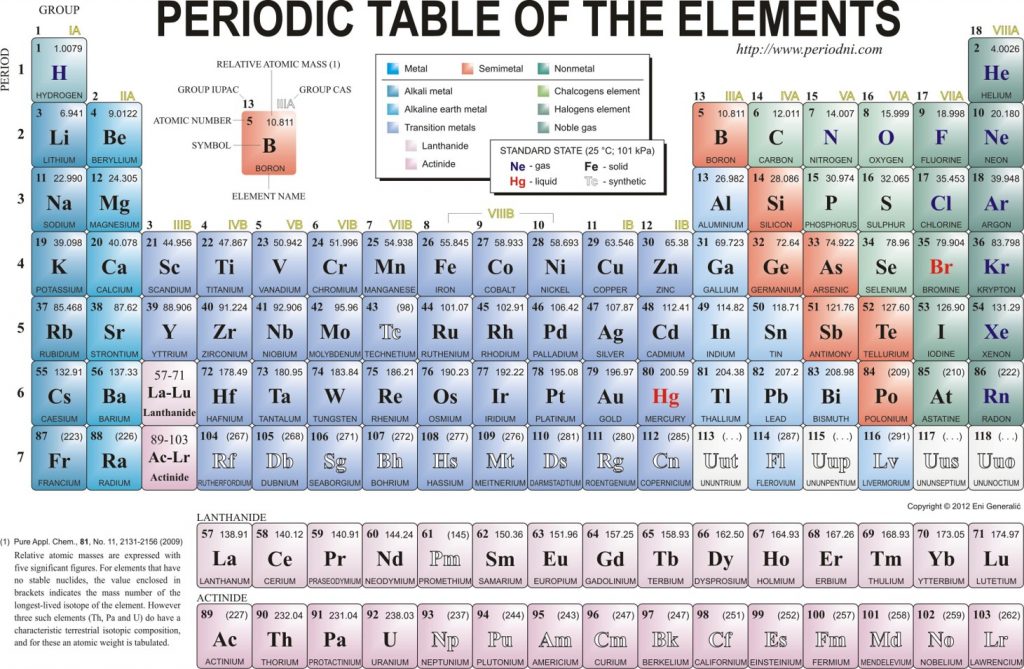
An element is a substance that cannot be broken down into simpler chemical substances. There are about 90 naturally occurring elements known on Earth. Using technology, scientists have been able to create nearly 30 additional elements that are not readily found in nature. Today, chemistry recognizes a total of 118 elements which are all represented on a standard chart of the elements, called the Periodic Table of Elements (Figure 2.1). Each element is represented by a one or two letter code, where the first letter is always capitalized and, if a second letter is present, it is written in lowercase. For example, the symbol for Hydrogen is H, and the symbol for carbon is C. Some of the elements have seemingly strange letter codes, such as sodium which is Na. These letter codes are derived from latin terminology. For example, the symbol for sodium (Na) is derived from the latin word, natrium, which means sodium carbonate.
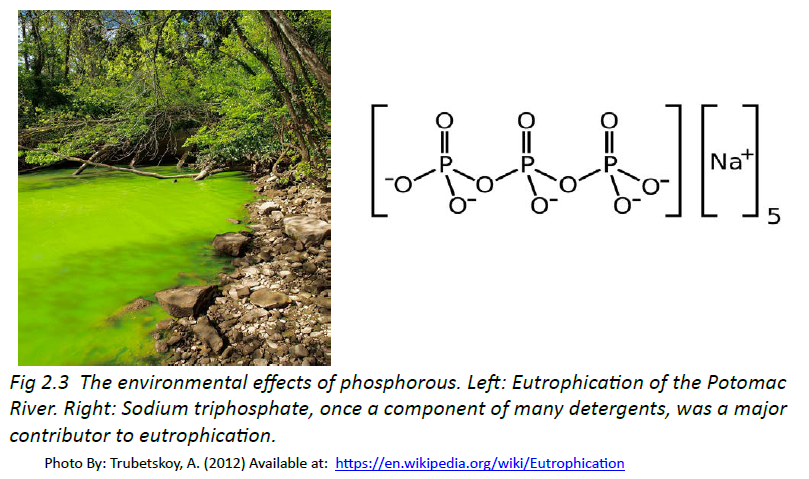
Figure 2.1: Elements. Some examples of pure elements include (A) Bismuth, Bi, a heavy metal is used as a replacement for lead and in some medicines, like pepto-bismol, the antidiarrheal and (B) Strontium, Sr, a major component in fireworks. (C) All of the elements that have been discovered are represented on the Periodic Table of Elements, which provides an elegant mechanism for not only displaying the elements, but describing many of their characteristics.
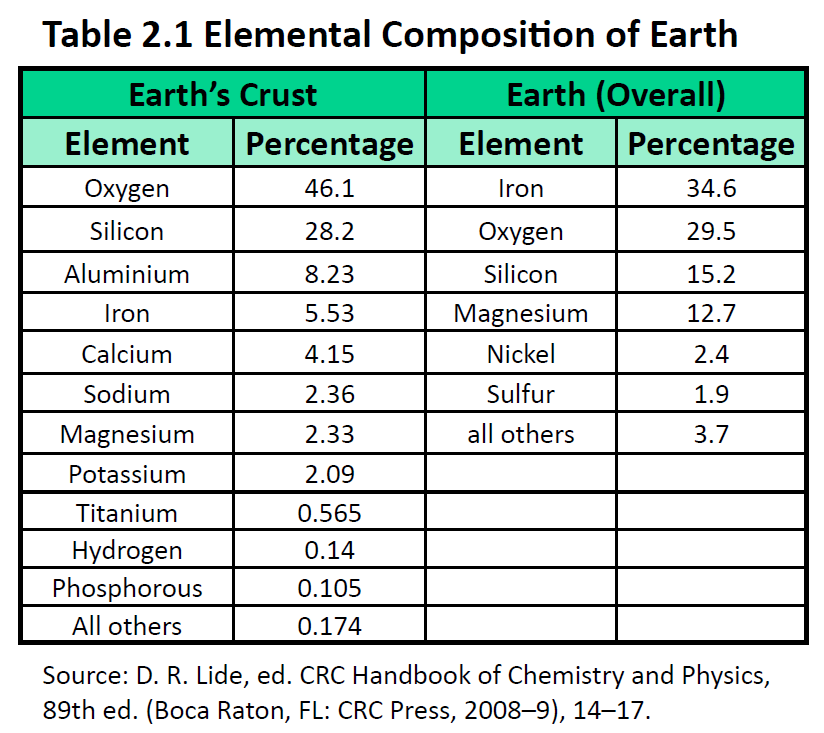
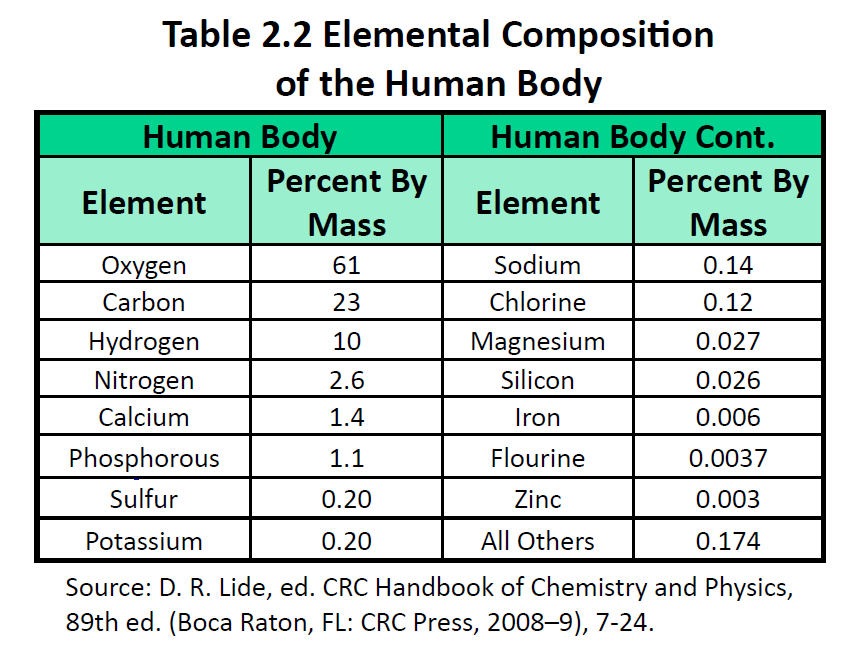
The elements vary widely in abundance. In the universe as a whole, the most common element is hydrogen (about 90%), followed by helium (most of the remaining 10%). All other elements are present in relatively minuscule amounts, as far as we can detect. On the planet Earth, however, the situation is rather different. Oxygen makes up 46.1% of the mass of Earth’s crust (the relatively thin layer of rock forming Earth’s surface), mostly in combination with other elements, while silicon makes up 28.5%. Hydrogen, the most abundant element in the universe, makes up only 0.14% of Earth’s crust. Table 2.1 “Elemental Composition of Earth” lists the relative abundances of elements on Earth as a whole and in Earth’s crust. Table 2.2 “Elemental Composition of a Human Body” lists the relative abundances of elements in the human body. If you compare Table 2.1 “Elemental Composition of Earth” and Table 2.2 “Elemental Composition of a Human Body”, you will find disparities between the percentage of each element in the human body and on Earth. Oxygen has the highest percentage in both cases, but carbon, the element with the second highest percentage in the body, is relatively rare on Earth and does not even appear as a separate entry in Table 2.1 “Elemental Composition of Earth”; carbon is part of the 0.174% representing “other” elements. How does the human body concentrate so many apparently rare elements?
The relative amounts of elements in the body have less to do with their abundances on Earth than with their availability in a form we can assimilate. We obtain oxygen from the air we breathe and the water we drink. We also obtain hydrogen from water. On the other hand, although carbon is present in the atmosphere as carbon dioxide, and about 80% of the atmosphere is nitrogen, we obtain those two elements from the food we eat, not the air we breathe.
Atomic Theory
The modern atomic theory, proposed about 1803 by the English chemist John Dalton, is a fundamental concept that states that all elements are composed of atoms. An atom is the smallest part of an element that maintains the identity of that element. Individual atoms are extremely small; even the largest atom has an approximate diameter of only 5.4 × 10−10 m. With that size, it takes over 18 million of these atoms, lined up side by side, to equal the width of your little finger (about 1 cm).
Most elements in their pure form exist as individual atoms. For example, a macroscopic chunk of iron metal is composed, microscopically, of individual iron atoms. Some elements, however, exist as groups of atoms called molecules. Several important elements exist as two-atom combinations and are called diatomic molecules. In representing a diatomic molecule, we use the symbol of the element and include the subscript 2 to indicate that two atoms of that element are joined together. The elements that exist as diatomic molecules are hydrogen (H2), oxygen (O2), nitrogen (N2), fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2).
Subatomic Particles
There have been several minor but important modifications to Dalton’s atomic theory. For one thing, Dalton considered atoms to be indivisible. We know now that atoms not only can be divided but also are composed of three different kinds of particles with their own properties that are different from the chemical properties of atoms.
The first subatomic particle was identified in 1897 and called the electron. It is an extremely tiny particle, with a mass of about 9.109 × 10−31 kg. Experiments with magnetic fields showed that the electron has a negative electrical charge.
By 1920, experimental evidence indicated the existence of a second particle. A proton has the same amount of charge as an electron, but its charge is positive, not negative. Another major difference between a proton and an electron is mass. Although still incredibly small, the mass of a proton is 1.673 × 10−27 kg, which is almost 2,000 times greater than the mass of an electron. Because opposite charges attract each other (while ‘like’ charges repel each other), protons attract electrons (and vice versa).
Finally, additional experiments pointed to the existence of a third particle, called the neutron. Evidence produced in 1932 established the existence of the neutron, a particle with about the same mass as a proton but with no electrical charge.
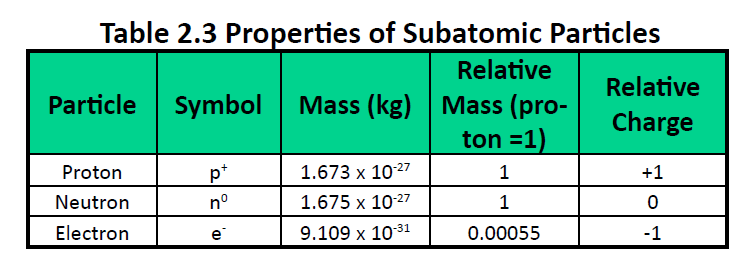
We understand now that all atoms can be broken down into subatomic particles: protons, neutrons, and electrons. Table 2.3 “Properties of the Subatomic Particles” lists some of their important characteristics and the symbols used to represent each particle. Experiment have shown that protons and neutrons are concentrated in a central region of each atom called the nucleus (plural, nuclei). Electrons are outside the nucleus and orbit about it because they are attracted to the positive charge in the nucleus. Most of the mass of an atom is in the nucleus, while the orbiting electrons account for an atom’s size. As a result, an atom consists largely of empty space. (Figure 2.4 and 2.5).
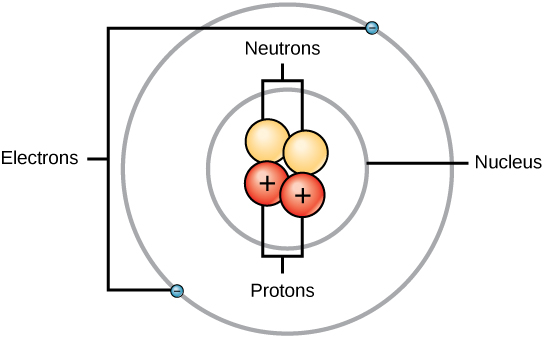
Fig 2.4 The anatomy of an atom. The protons and neutrons of an atom are found clustered at the center of the atom in a structure called the nucleus. The electrons orbit the nucleus of the atom within an electron cloud, or the empty space that surrounds the atom’s nucleus. Note that most of the area of an atom is taken up by the empty space of the electron cloud.
Source: https://upload.wikimedia.org/wikipedia/commons/2/24/Figure_02_01_01.jpg
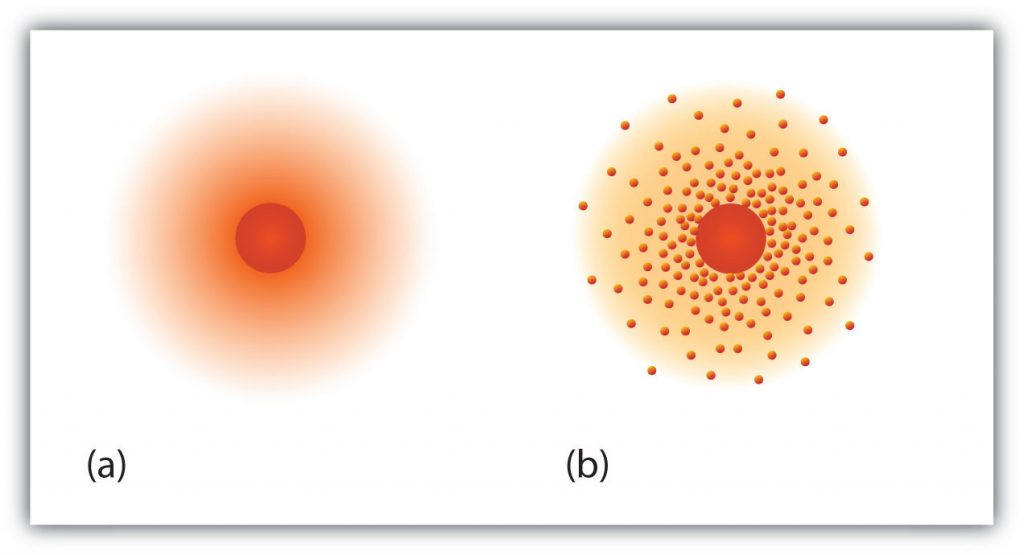
Fig 2.5 The path of the electron in a hydrogen atom. Electrons are not in discrete orbits like planets around the sun. Instead there is a probability that an electron may occupy a certain space within the electron cloud (a) The darker the color, the higher the probability that the hydrogen’s one electron will be at that point at any given time. (b) Similarly, the more crowded the dots, the higher the probability that hydrogen’s one electron will be at that point. In both diagrams, the nucleus is in the center of the diagram.
Protons Determine the Identity of an Element
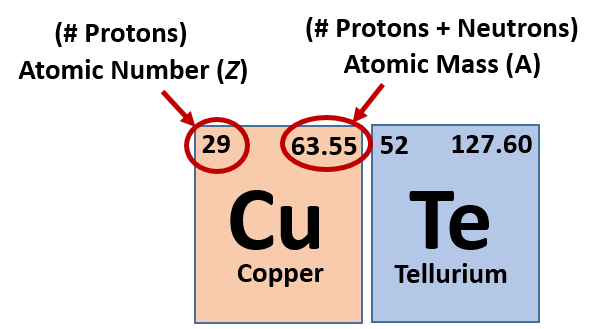
As it turns out, the number of protons that an atom holds in its nucleus is the key determining feature for its chemical properties. In short, an element is defined by the number of protons found in its nucleus. The proton number within an element is also called its Atomic Number and is represented by the mathematical term, Z (Fig 2.6). If you refer back to the Periodic Table of Elements shown in figure 2.1, you will see that the periodic table is organized by the number of protons that an element contains. Thus, as you read across each row of the Periodic Table (left to right), each element increases by one proton (or one Atomic Number, Z).
Fig 2.6 Structure of the Periodic Table. Each element on the periodic table is represented by the atomic symbol (Cu for Copper), the Atomic Number in the upper lefthand corner, and the Atomic Mass in the righthand corner.
Isotopes, Allotropes, and Atomic Mass
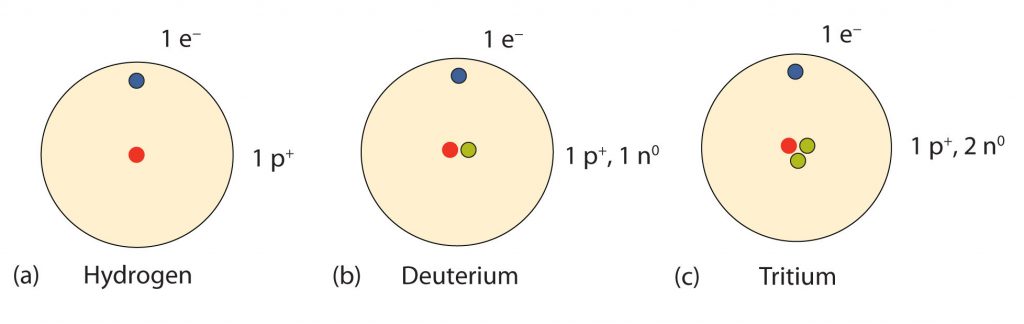
How many neutrons are in atoms of a particular element? At first it was thought that the number of neutrons in a nucleus was also characteristic of an element. However, it was found that atoms of the same element can have different numbers of neutrons. Atoms of the same element that have different numbers of neutrons are called isotopes (Fig. 2.7). For example, 99% of the carbon atoms on Earth have 6 neutrons and 6 protons in their nuclei; about 1% of the carbon atoms have 7 neutrons and 6 protons in their nuclei. Naturally occurring carbon on Earth, therefore, is actually a mixture of isotopes, albeit a mixture that is 99% carbon with 6 neutrons in each nucleus. Isotope composition has proven to be a useful method for dating many rock layers and fossils.
Fig 2.7 Isotopes of Hydrogen. All hydrogen atoms have one proton and one electron. However, they can differ in the number of neutrons. (a) Most hydrogen atoms onlycontain one p+ and one e- and no neutrons (b) A small amount of hydrogen exists as the isotope deuterium, which has one proton and one neutron in its nucleus, and (c) an even smaller amount contains one proton and two neutrons in its nucleus and is termed Tritium. Note that Tritium is unstable isotope and will breakdown over time. Thus, Tritium is a radioactive element.
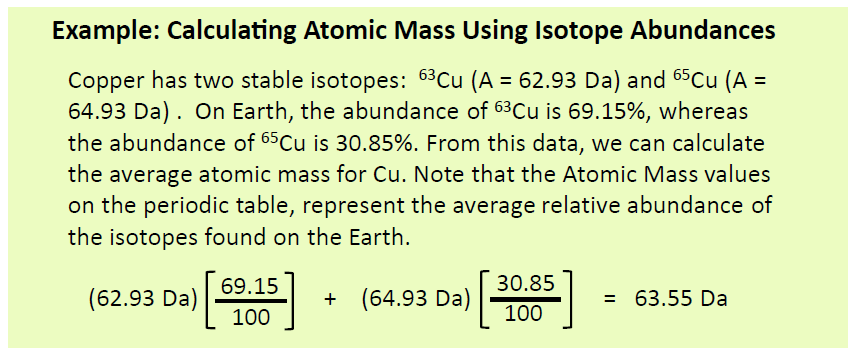
Most elements exist as mixtures of isotopes. In fact, there are currently over 3,500 isotopes known for all the elements. When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. The atomic mass (A) of an atom is the sum of the numbers of protons and neutrons in the nucleus (Fig. 2.6). Given the atomic mass for a nucleus (and knowing the atomic number, Z, of that particular atom), you can determine the number of neutrons by subtracting the atomic number from the atomic mass.
A simple way of indicating the mass number of a particular isotope is to list it as a superscript on the left side of an element’s symbol. Atomic numbers are often listed as a subscript on the left side of an element’s symbol. Thus, we might see
which indicates a particular isotope of copper. The 29 is the atomic number, Z, (which is the same for all copper atoms), while the 63 is the atomic mass (A) of the isotope. To determine the number of neutrons in this isotope, we subtract 29 from 63: 63 − 29 = 34, so there are 34 neutrons in this atom.
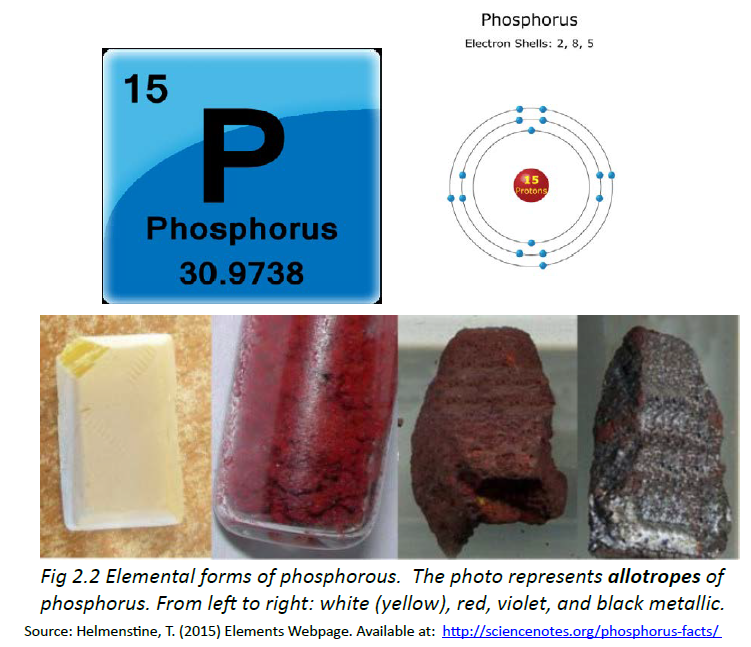
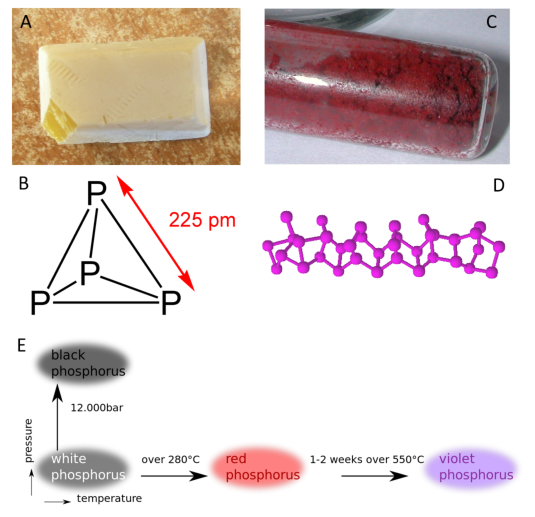
Allotropes of an element are different and separate from the term isotope and should not be confused. Some chemical elements can form more than one type of structural lattice, these different structural lattices are known as allotropes. This is the case for phosphorus as shown in Figure 2.2. White or yellow phosphorus forms when four phosphorus atoms align in a tetrahedral conformation (Fig 2.8). The other crystal lattices of phosphorus are more complex and can be formed by exposing phosphorus to different temperatures and pressures. For example, the cage-like lattice of red phosphorus can be formed by heating white phosphorus over 280oC (Fig 2.8). Note that allotropic changes affect how the atoms of the element interact with one another to form a 3-dimensional structure. They do not alter the sample with regard to the atomic isotope forms that are present, and DO NOT alter or affect the atomic mass (A) of the element.
Different allotropes of different elements can have different physical and chemical properties and are thus, still important to consider. For example, oxygen has two different allotropes with the dominant allotrope being the diatomic form of oxygen, O2. However, oxygen can also exist as O3, ozone. In the lower atmosphere, ozone is produced as a by-product in automobile exhaust, and other industrial processes where it contributes to pollution. It has a very pungent smell and is a very powerful oxidant. It can cause damage to mucous membranes and respiratory tissues in animals. Exposure to ozone has been linked to premature death, asthma, bronchitis, heart attacks and other cardiopulmonary diseases. In the upper atmosphere, it is created by natural electrical discharges and exists at very low concentrations. The presence of ozone in the upper atmosphere is critically important as it intercepts very damaging ultraviolet radiation from the sun, preventing it from reaching the Earth’s surface.
Figure 2.8 Allotropes of Phosphorus. (A) White phosphorus exists as a (B) tetrahedral form of phosphorus, whereas (C) red phosphorus has a more (D) cage-like crystal lattice. (E) The different elemental forms of phosphorus can be created by treating samples of white phosphorus with increasing temperature and pressure.
Source: https://en.wikipedia.org/wiki/Allotropes_of_phosphorus
Electrons and the Periodic Table of the Elements
Remember that electrons are 2000 times smaller than protons and yet each one contains an equal, but opposing charge. Electrons have a negative charge while protons have a positive charge. Interestingly, when elements exist in their elemental form, as shown on the periodic table, the number of electrons housed in an atom is equal to the number protons. Therefore, the electric charge of an element cancels itself out and the overall charge of the atom is zero.
Electrons are the mobile part of the atom. They move and orbit the nucleus of the atom in the electron cloud, the term used for the space around the nucleus. However, they do not move around in random patterns. Electrons have addresses, or defined orbital spins, within the electron cloud, much the same way our apartment buildings have addresses within our cities. To find the address of an electron, you need to know a little bit about the organization of the electron cloud (…or the city that the electron lives in).
The electron cloud of an atom is divided into layers, called shells, much the way an onion has layers when you peel it. However, it is incorrect to think of a shell as a single layer without thickness and depth to it. A shell has 3-dimensional space within it that contains a wide variety of ‘apartments’ or spaces for the electrons to occupy. Thus, the shell, or n number, is only the first part of an electron’s address within an atom. It would be similar to only knowing the neighborhood where your friend lives. If you only know the neighborhood, it will be difficult to find your friend if you want to take them to dinner.
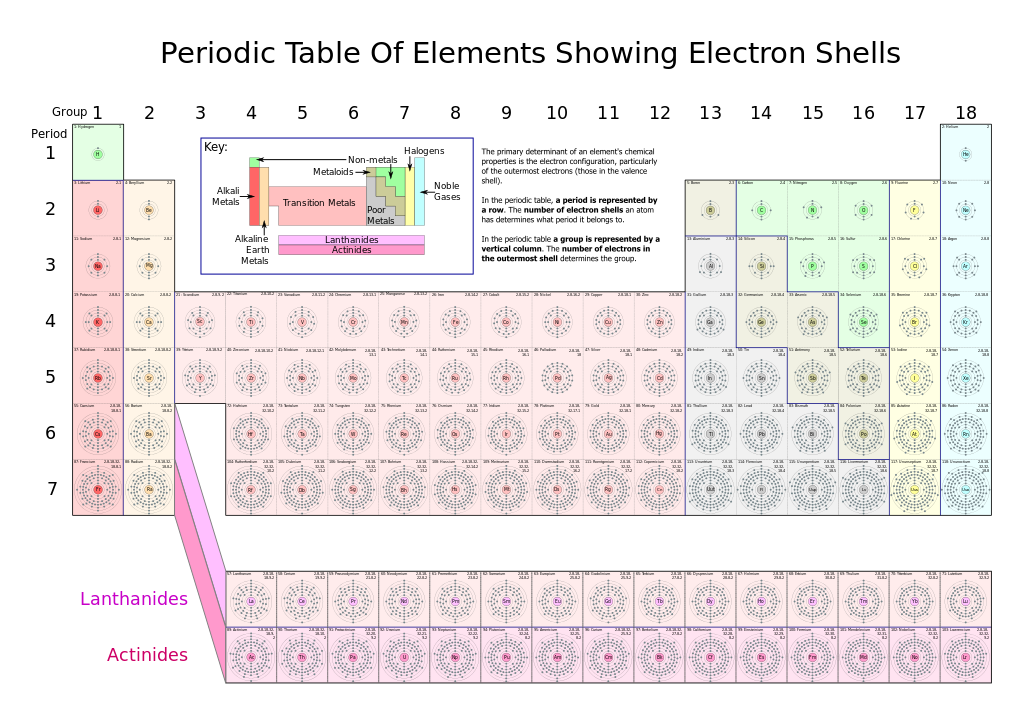
There are a total of 7 shells (or layers) that an atom can have to house it’s electrons. If an atom is small, it may only have 1 or 2 shells. Only very large atoms have all 7 layers. After this point, adding an 8th shell appears to make the atom too unstable to exist…at least we have never found atoms containing an 8th shell! In the periodic table (Fig. 2.9), you will notice that there are a total of 7 rows on the periodic table (note that the Lanthanide and Actinide rows of elements are generally shown below the main table to make them fit onto one page, but they really belong in the middle of rows 6 and 7 on the periodic table, according to their atomic numbers). Each of these rows represents an electron shell. Thus, as atoms get larger and house more electrons, they acquire additional shells, up to 7.
Fig 2.9 Structure of the Periodic Table. Each element on the periodic table is represented by the atomic symbol (Cu for Copper), the Atomic Number in the upper lefthand corner, and the Atomic Mass in the righthand corner.
Source: Robson, G.(2006) Wikipedia. https://en.wikipedia.org/wiki/Electron_shell
Within this textbook, we are not concerned with learning the addresses of all the electrons, but we are very interested about the electrons that are nearest to the surface of the atom, or the ones that are in the outer shell of the atom. The electrons that are closest to the surface of the atom are the most reactive and are integral in forming bonds between the atoms. These electrons are said to be housed in the atom’s, valence shell, or the electron shell that is the farthest away from the nucleus of the atom. (or nearest to the surface of the atom).
Features of the Periodic Table
Elements that have similar chemical properties are grouped in columns called groups (or families). As well as being numbered, some of these groups have names—for example, alkali metals (the first column of elements), alkaline earth metals (the second column of elements), halogens (the next-to-last column of elements), and noble gases (the last column of elements).
Each row of elements on the periodic table is called a period. Periods have different lengths; the first period has only 2 elements (hydrogen and helium), while the second and third periods have 8 elements each. The fourth and fifth periods have 18 elements each, and later periods are so long that a segment from each is removed and placed beneath the main body of the table.
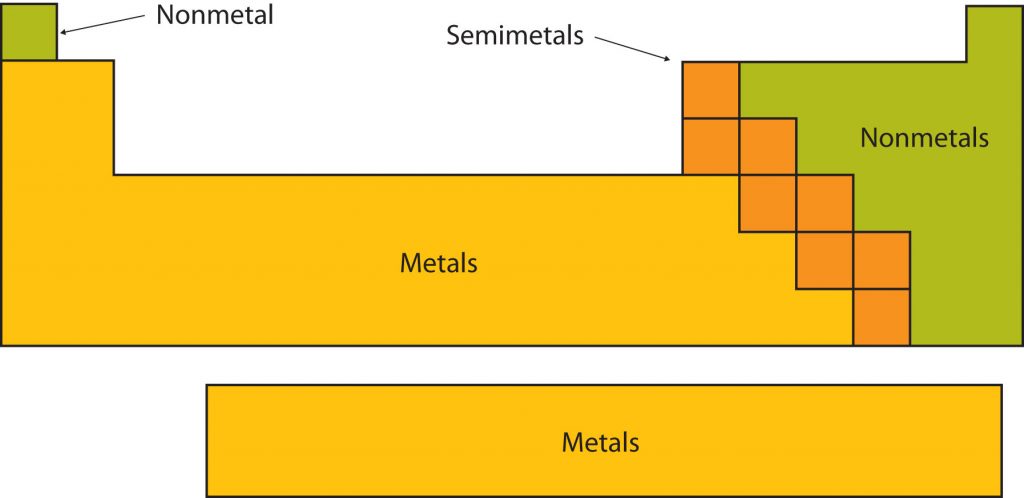
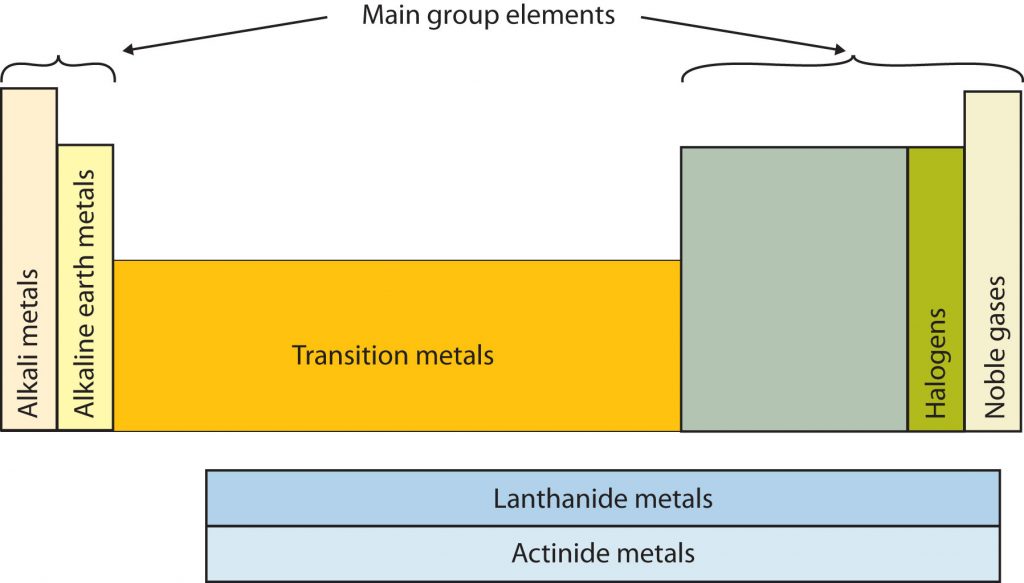
Certain elemental properties become apparent in a survey of the periodic table as a whole. Every element can be classified as either a metal, a nonmetal, or a semimetal, as shown in Figure 2.10 “Types of Elements”. A metal is a substance that is shiny, typically (but not always) silvery in color, and an excellent conductor of electricity and heat. Metals are also malleable (they can be beaten into thin sheets) and ductile (they can be drawn into thin wires). A nonmetal is typically dull and a poor conductor of electricity and heat. Solid nonmetals are also very brittle. As shown in Figure 2.7 “Types of Elements”, metals occupy the left three-fourths of the periodic table, while nonmetals (except for hydrogen) are clustered in the upper right-hand corner of the periodic table. The elements with properties intermediate between those of Another way to categorize the elements of the periodic table is shown in Figure 2.11 “Special Names for Sections of the Periodic Table”. The first two columns on the left and the last six columns on the right are called the main group elements. The ten-column block between these columns contains the transition metals. The two rows beneath the main body of the periodic table contain the inner transition metals. The elements in these two rows are also referred to as, respectively, the lanthanide metals and the actinide metals (Fig 2.11).
Fig 2.10. Types of Elements. Elements are either metals, nonmetals, or semimetals. Each group is located in a different part of the periodic table.
Fig 2.11. Special Names for Sections of the Periodic Table. Some sections of the periodic table have special names. For example, the elements lithium, sodium, potassium, rubidium, cesium, and francium are collectively known as alkali metals. Note that the main group elements do not include the transition metals.
The periodic table is organized on the basis of similarities in elemental properties, but what explains these similarities? It turns out that the arrangement of the columns or families in the Periodic Table reflects how subshells are filled with electrons. Of note, elements in the same column share the same valence shell electron configuration. For example, all elements in the first column have a single electron in their valence shells. This last observation is crucial. Chemistry is largely the result of interactions between the valence electrons of different atoms. Thus, atoms that have the same valence shell electron configuration will have similar chemistry (Fig 2.12).
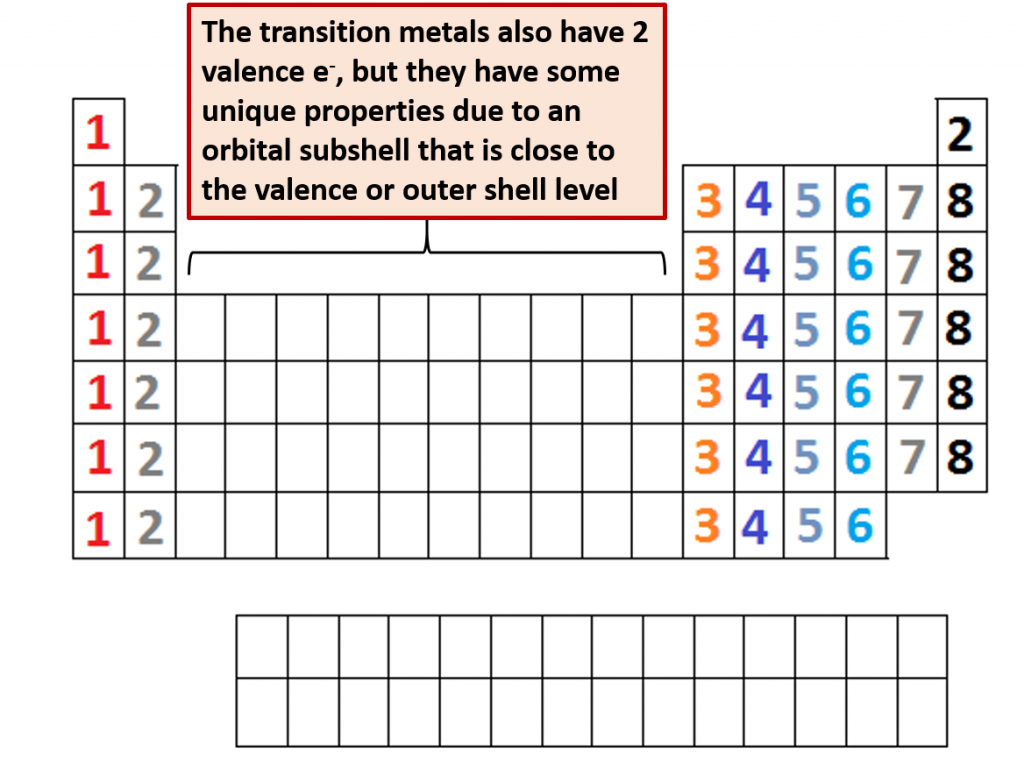
Fig 2.12. Number of Valence Shell Electrons. The placement of elements on the periodic table corresponds with the number of valence electrons housed in that element. Families (columns) on the periodic table all contain the same number of valence shell electrons, which gives them similar chemical properties and reactivities. You can easily count across the main group elements to see the increasing number of electrons in the valence shell. All of the transition metals have 2 e- in their valence shell, although they also contain an inner orbital subshell that is very close to the valence shell. This gives some of these metals different levels of reactivity. Note that the maximum number of valence shell electrons possible is 8, and that is obtained only by the Noble Gases.
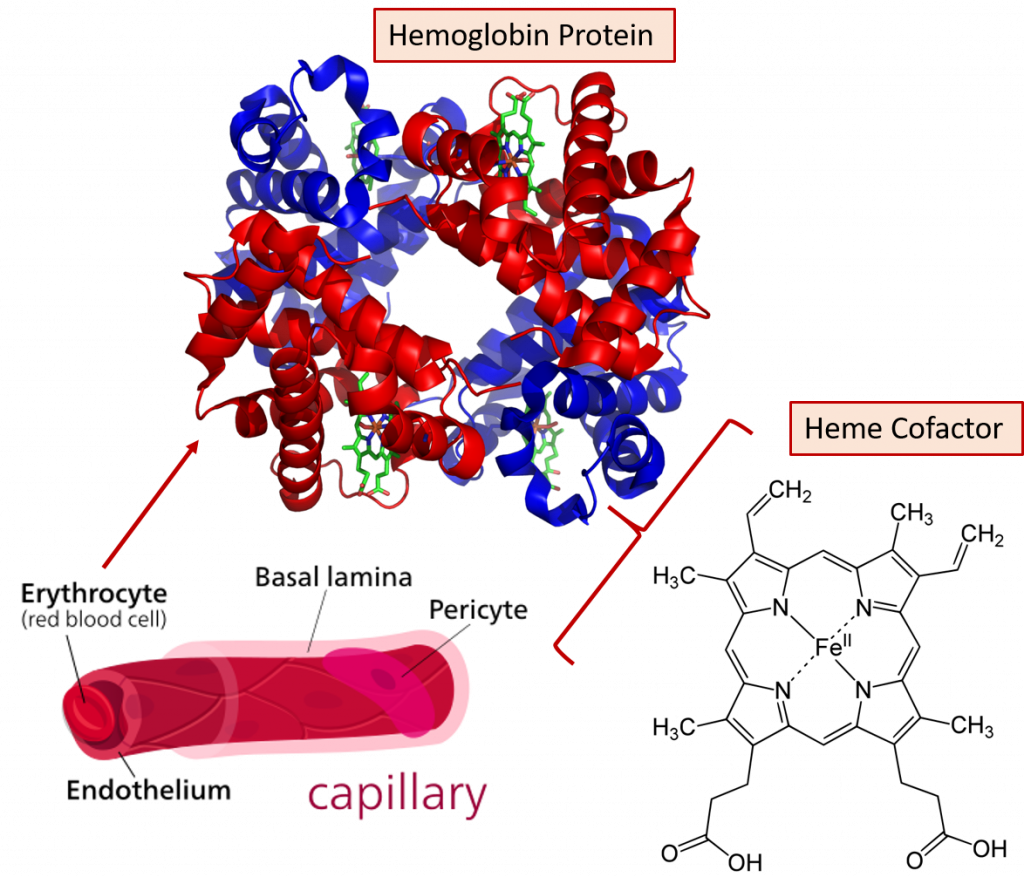
Fig 2.13. Role of iron in oxygen transportation. The hemoglobin protein makes up about 95% of the dry content of the red blood cell and each hemoglobin protein can bind and carry four molecules of oxygen (O2).
Adapted from: https://en.wikipedia.org/wiki/Hemoglobin and https://en.wikipedia.org/wiki/Capillary
2.3 Chapter Summary
2.4 References
Chapter 2 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf