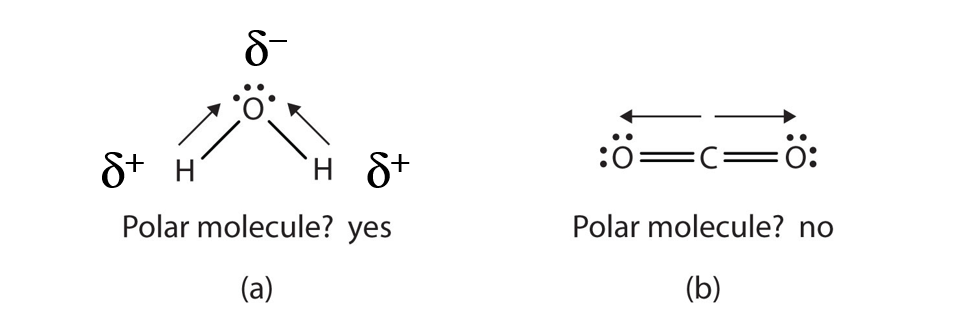Home » Student Resources » Online Chemistry Textbooks » CH103: Allied Health Chemistry » CH103 – Chapter 5: Covalent Bonds and Introduction to Organic Molecules
MenuCH103: Allied Health Chemistry
Chapter 5: Covalent Bonds and Introduction to Organic Molecules
This text is published under creative commons licensing, for referencing and adaptation, please click here.
5.1 Introduction to Covalent Molecules and Compounds
How to Recognize Covalent Bonds
5.2 Electron Sharing
Single Covalent Bonds Between the Same Atoms
Single Covalent Bonds Between Different Atoms
Multiple Covalent Bonds
Coordinate Covalent Bonds
5.3 Electronegativity and Bond Polarity
5.4 Properties of Molecular Compounds
5.5 Naming Binary Molecular Compounds
5.6 Intermolecular Forces
5.7 Recognizing and Drawing Organic Molecules
5.8 Stereoisomers, Enantiomers, and Chirality
5.9 The Importance of Chirality in Protein Interactions
5.10 Common Organic Functional Groups
5.11 Chapter Summary
5.12 References
Chapter 5 – Covalent Bonds and Introduction to Organic Molecules
Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds represent extreme cases that are seldom encountered in any but very simple substances, a brief discussion of these two extremes helps explain why substances with different kinds of chemical bonds have very different properties. Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist of molecules, which are groups of atoms in which one or more pairs of electrons are shared between bonded atoms. In a covalent bond, atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share. This chapter will focus on the properties of covalent compounds.
5.1 Introduction to Covalent Molecules and Compounds
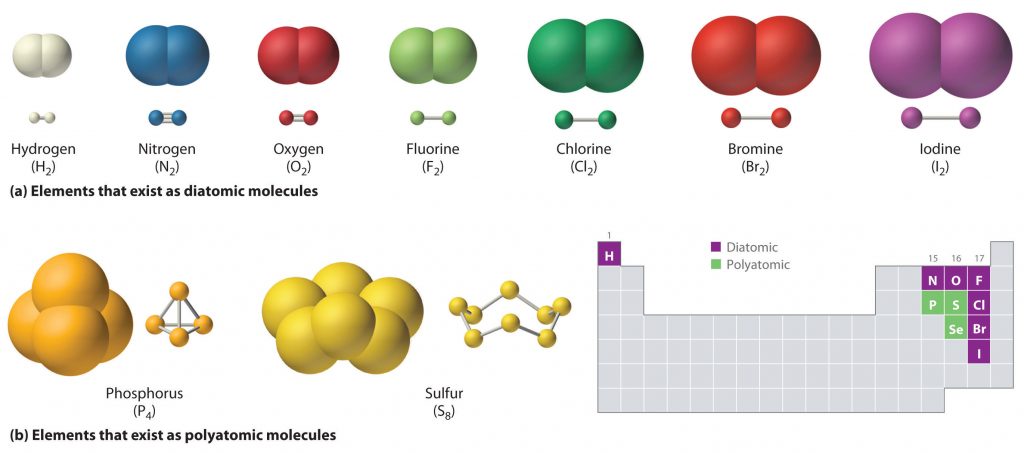
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the fundamental chemical properties of a covalent compound. Thus, the term molecular compound is used to describe elements that are covalently bonded and to distinguish the compounds from ionic compounds. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H2, N2, O2, F2, Cl2, Br2, and I2 (part (a) in Figure 4.1). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, such as elemental phosphorus and sulfur, which occur as P4 and S8 (part (b) in Figure 5.1).
Figure 5.1 Elements That Exist as Covalent Molecules. (a) Several elements naturally exist as diatomic molecules, in which two atoms (E) are joined by one or more covalent bonds to form a molecule with the general formula E2. (b) A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedra—regular polyhedra with four triangular sides—with a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
Each covalent compound is represented by a molecular formula, which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1. For example, water, with two hydrogen atoms and one oxygen atom per molecule, is written as H2O. Similarly, carbon dioxide, which contains one carbon atom and two oxygen atoms in each molecule, is written as CO2.
Covalent compounds that contain carbon and hydrogen are called organic compounds. The convention for representing the formulas of organic compounds is to write carbon first, followed by hydrogen and then any other elements in alphabetical order (e.g., CH4O is methyl alcohol, a fuel). Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds; they include both covalent and ionic compounds. The convention for writing inorganic compounds, involves listing the component elements beginning with the one farthest to the left in the periodic table, as in SO2 or SF6. Those in the same group are listed beginning with the lower element and working up, as in ClF. By convention, however, when an inorganic compound contains both hydrogen and an element from groups 13–15, hydrogen is usually listed last in the formula. Examples are ammonia (NH3) and silane (SiH4). Compounds such as water, whose compositions were established long before this convention was adopted, are always written with hydrogen first: Water is always written as H2O, not OH2. Typically this distinguishes when hydrogen is participating in a covalent bond rather than an ionic interaction, as seen in many of the inorganic acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), as described in chapter 4.
How to Recognize Covalent Bonds
In Chapter 4, we saw that ionic compounds are composed predominantly of a metal + a nonmetal. Covalent molecules, on the otherhand, are typically composed of two nonmetals or a nonmetal and a metalloid. This is an initial screening method that you can use to categorize compounds into the ionic or the covalent cagetogy.
Figure 5.2 Recognizing Ionic vs Covalent Compounds. Typically compounds that are formed from a combination of a metal with a nonmetal have more ionic bond character whereas compounds formed from two nonmetals or a metalloid and a nonmetal show more covalent character. Although compounds usually lie on a spectrum somewhere between fully ionic and fully covalent character, for naming purposes, this guideline works well.
(back to the top)
5.2 Electron Sharing
Predicting the Correct Number of Bonds
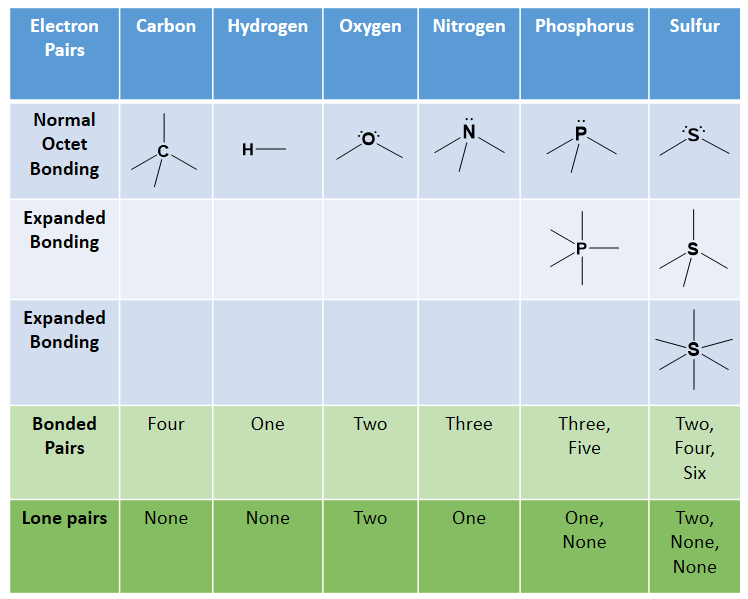
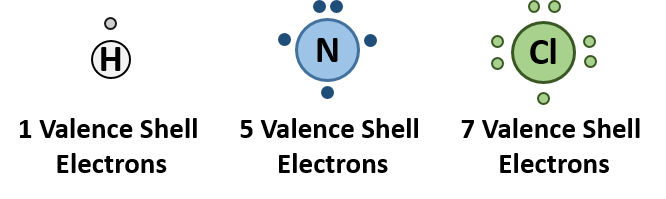
Recall that the octet rule helped us determine that carbon has four electrons in its valence shell and would thus, need to create four covalent bonds to reach an octet. Similarly, nitrogen and phosphorus each make three bonds, oxygen and sulfur each make two, and the halogens only make one bond. Hydrogen is an exception to the octet rule as it is the smallest element and its valence shell is filled with two electrons. Thus, hydrogen can only form one bond with another atom. Sulfur and phosphorus can also have bonding patterns that are exceptions to the octet rule. They both can have expanded orbital bonding with phosphorus also routinely forming five covalent bonds, and sulfur being capable of forming either four or six covalent bonds. Table 5.1 provides a graphic representation of these patterns. When you are drawing organic molecules, it is important to pay attention to the bonding rules so that all atoms reach their preferred bonding states.
Table 5.1: Covalent Bonding Patterns of Atoms Commonly Atoms
*Note: Hydrogen doesn’t really follow the octet rule as its valence shell is full with 2 e–
Single Covalent Bonds Between the Same Atoms
Chapter 4 described how electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell following the octet rule. However, there is another way an atom can achieve a full valence shell: atoms can share electrons to reach the octet state (or the duet state in the case of hydrogen).
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) We can represent the two individual hydrogen atoms as follows:
In this situation neither hydrogen can reach the preferred duet state. In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. In this configuration, each hydrogen has an electron configuration equivalent to that of the noble gas, helium. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. For example, one molecule of water would contain two hydrogen atoms and one oxygen atom (H2O).
Chemists frequently use Lewis electron dot diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
The Lewis diagram of two hydrogen atoms sharing electrons looks like this:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
Remember that the dash, also referred to as a single bond, represents a pair of bonding electrons.
The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10−11 m, or 74 picometers (pm; 1 pm = 1 × 10−12 m). This particular bond length represents a balance between several forces: (1) the attractions between oppositely charged electrons and nuclei, (2) the repulsion between two negatively charged electrons, and (3) the repulsion between two positively charged nuclei. If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following electron dot diagrams:
Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule:
The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
(back to the top)
Single Covalent Bonds Between Different Atoms
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:
Each atom needs one additional electron to complete its valence shell. By each contributing one electron, they make the following molecule:
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the valence electron shells are filled for both atoms (recall that hydrogen is filled with two electrons).
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH4), with one carbon atom and four hydrogen atoms, can be represented as follows:
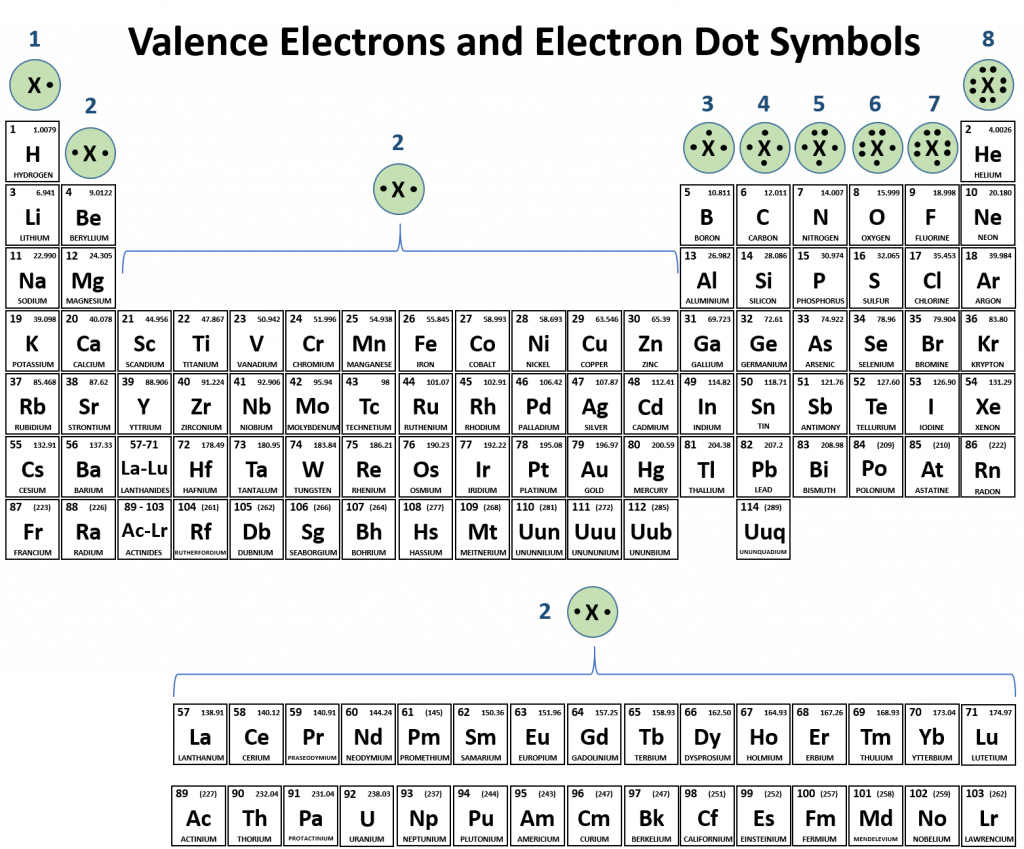
Atoms typically form a characteristic number of covalent bonds in compounds. Figure 5.3 shows valence electron configurations of each element family (or column).
Fig 5.3 Periodic Table with Lewis Structures. Each family shows a representative lewis structure for that group of elements. For the nonmetals (Families 4A, 5A, 6A, and 7A) they can accept a complementary number of shared bonds to reach the octet state. Family 4A can share 4 covalent bonds (4 + 4 = 8), whereas Families 5A, 6A, and 7A can share 3, 2, and 1 covalent bond(s), respectively, to achieve the octet state. Exceptions to the octet rule do exist. For example, hydrogen can be considered to be in Group 1 or Group 7A because it has properties similar to both groups. Hydrogen can participate in either ionic or covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has one electron to start with, it can only make one covalent bond. Similarly, boron has 3 electrons in its outer shell. This nonmetal typically forms 3 covalent bonds, having a maximum of 6 electrons in its outer shell. Thus, boron can never reach the octet state. Other atoms can have expanded orbitals and accept additional covalent bonds. Two of these that are important for living systems are sulfur and phosphorus. By the octet rule, sulfur can make 2 covalent bonds and phosphorus 3 covalent bonds. Sulfur can also have expanded orbitals to accept 4 or 6 covalent bonds, and phosphorus can expand to 5 covalent bonds.
(back to the top)
Multiple Covalent Bonds
In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons. Consider carbon dioxide (CO2). If each oxygen atom shares one electron with the carbon atom, we get the following:
This does not give either the carbon or oxygen atoms a complete octet; The carbon atom only has six electrons in its valence shell and each oxygen atom only has seven electrons in its valence shell. Thus, none of the atoms can reach the octet state in the current configuration. As written, this would be an unstable molecular conformation.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C2H2), whose Lewis diagram is as follows:
Coordinate Covalent Bonds
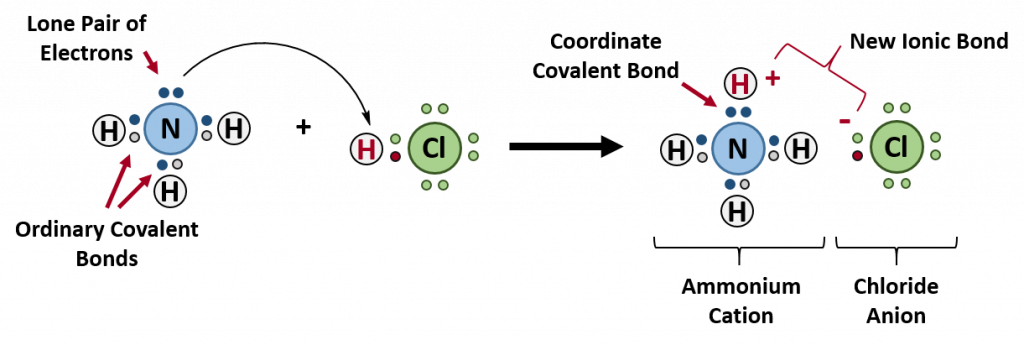
A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. In the formation of a simple or ordinary covalent bond, each atom supplies one electron to the bond – but that does not have to be the case. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons. The following reaction between ammonia and hydrochloric acid demonstrates the formation of a coordinate covalent bond between ammonia and a hydrogren ion (proton).
The reaction between ammonia and hydrochloric acid
If these colorless gases are allowed to mix, a thick white smoke of solid ammonium chloride is formed.
The overall reaction is
Ammonium ions, NH4+, are formed by the transfer of a hydrogen ion (a proton) from the hydrochloric acid molecule to the lone pair of electrons on the ammonia molecule. To visualize this reaction, we can use electron dot configurations to observe the electron movement during the reaction. First recall the valence electron states for all of the atoms involved in the reaction:
On the left side of the equation (to the left of the arrow) are the reactants of the reaction (ammonia and hydrochloric acid). On the right side of the reaction (to the right of the arrow) is the product of the reaction, the ionic compound – ammonium chloride. The diagram below shows the electron and proton movement during the reaction.
Figure 5.4 Formation of Ammonium Chloride. When the ammonium ion, NH4+, is formed, the fourth hydrogen (shown in red) is attached by a coordinate covalent bond, because only the hydrogen’s nucleus is transferred from the chlorine to the nitrogen. The hydrogen’s electron is left behind on the chlorine to form a negative chloride ion. Once the ammonium ion has been formed it is impossible to tell any difference between the coordinate covalent and the ordinary covalent bonds, all of the hydrogens are equivalent in the molecule and the extra positive charge is distributed throughout the molecule. Although the electrons are shown differently in the diagram, there is no difference between them in reality. In simple diagrams, a coordinate bond is shown by a curved arrow. The arrow points from the atom donating the lone pair to the atom accepting it.
(back to the top)
5.3 Electronegativity and Bond Polarity
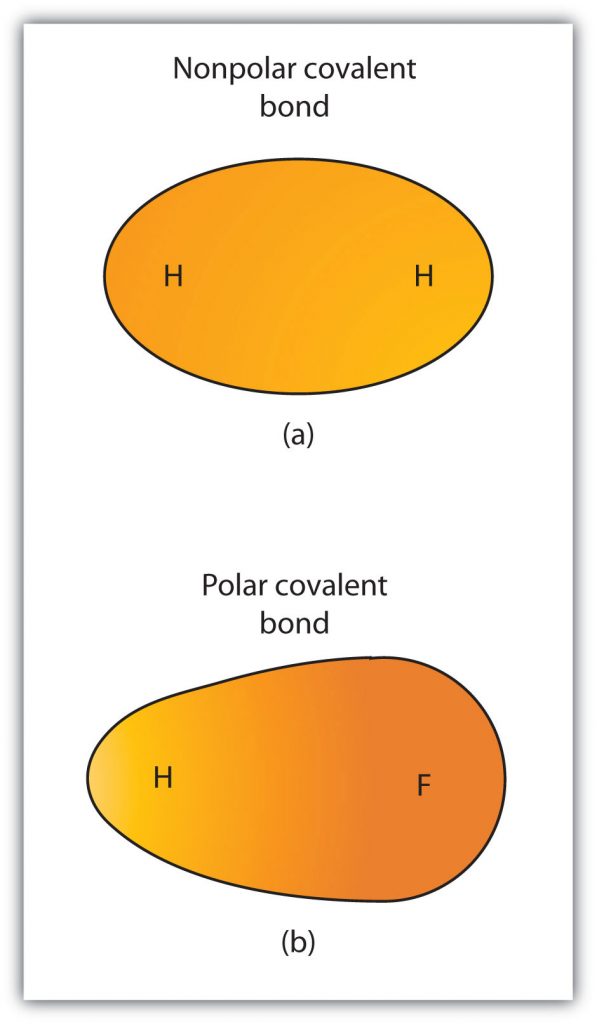
Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. Unless the bond connects two atoms of the same element, there will always be one atom that attracts the electrons in the bond more strongly than the other atom does, as shown in Figure 5.5. When such an imbalance occurs, there is a resulting buildup of some negative charge (called a partial negative charge and designated δ−) on one side of the bond and some positive charge (designated δ+) on the other side of the bond. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure 5.5, is called a polar covalent bond. A covalent bond that has an equal sharing of electrons (part (a) of Figure 5.5) is called a nonpolar covalent bond.
Figure 5.5 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. This is a nonpolar covalent bond. (b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. This is a polar covalent bond.
Video Tutorial on Nonpolar Covalent Bonds
Video Tutorial On Polar Covalent Bonds
Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred completely rather than shared. To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond.
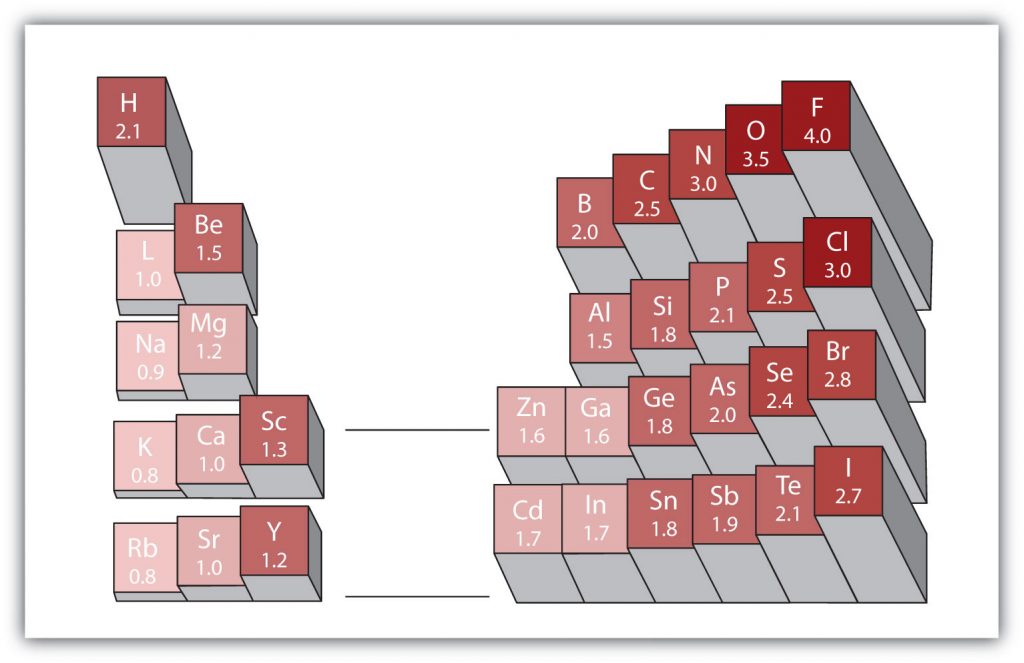
There are various numerical scales for rating electronegativity. Figure 5.6 shows one of the most popular—the Pauling scale. The polarity of a covalent bond can be judged by determining the difference in the electronegativities between the two atoms making the bond. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond.
Figure 5.6 Electronegativities of Various Elements. The Pauling Scale for electronegativities has the value for fluorine atoms set at 4.0, the highest value.
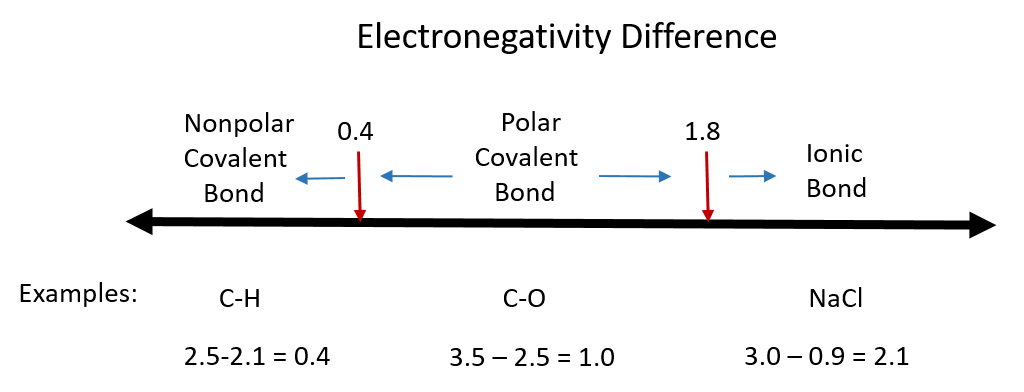
Although there are no hard and fast rules, the general rule is that a difference in electronegativity less than 0.4 indicates the bond is nonpolar; when the difference is greater than 0.4, the bond is considered polar. When the difference in electronegativities is large enough (generally greater than about 1.8), the resulting compound is considered ionic rather than covalent. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond. Examples of electronegativity difference are shown in Figure 5.7.
Figure 5.7 Electronegativity Difference Diagram. The diagram above is a guide for discerning what type of bond forms between two different atoms. By taking the difference between the electronegativity values for each of the atoms involved in the bond, the bond type and polarity can be predicted. Note that full ionic character is rarely reached, however when metals and nonmetals form bonds, they are named using the rules for ionic bonding.
When a molecule’s bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. For example, the orientation of the two O–H bonds in a water molecule (Figure 5.8) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties. (For example, the boiling point of water [100°C] is high for such a small molecule and is due to the fact that polar molecules attract each other strongly.) In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other in the molecule and so cancel each other’s effects. Thus, carbon dioxide molecules are nonpolar overall. This lack of polarity influences some of carbon dioxide’s properties. (For example, carbon dioxide becomes a gas at −77°C, almost 200° lower than the temperature at which water boils.)
Figure 5.8 Physical Properties and Polarity. The physical properties of water (a) and carbon dioxide (b) are affected by their molecular polarities. Note that the arrows in the diagram always point in the direction where the electrons are more strongly attracted. In this diagram, the delta symbol (δ) is used with a (+) or (-) symbol to represent partial positive and partial negative charge distribution in polar covalent bonds. Note that the electrons shared in polar covalent bonds will be attracted to and spend more time around the atom with the higher electronegativity value. When the polarity is equal and directly opposing, as in the case of carbon dioxide (b), the overall molecule will have no overall charge.
Tutorial on Electronegativity and Bond Polarity By Professor Dave Explains
5.4 Properties of Molecular Compounds
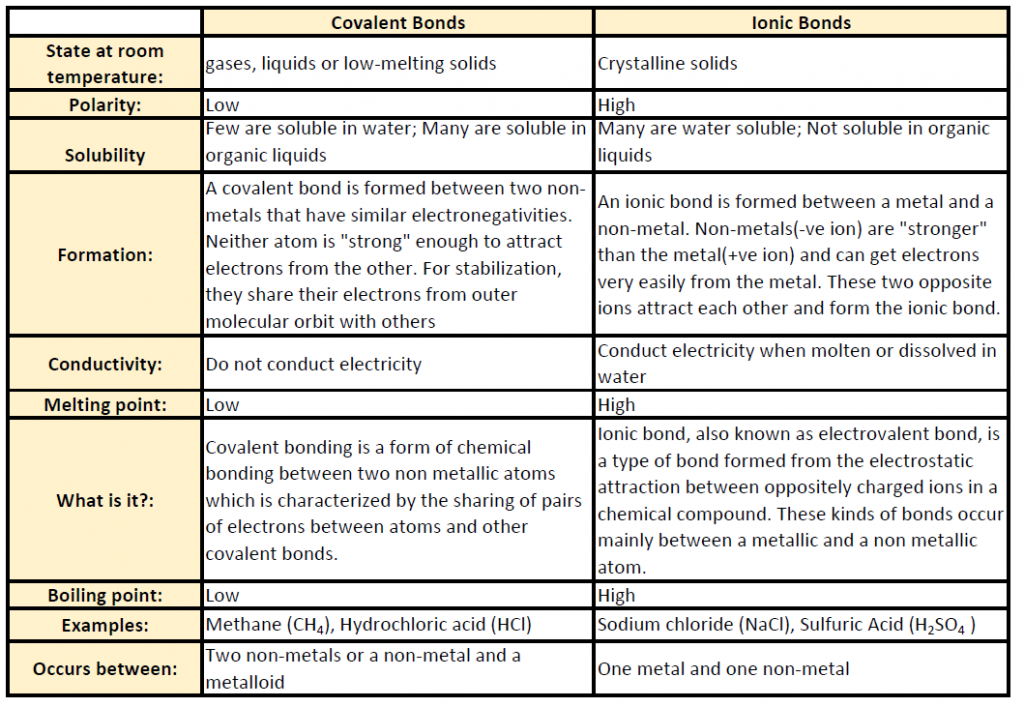
Molecular compounds have many properties that differ from ionic compounds. Some of the generalizations for this group include much lower melting and boiling points when compared with their ionic counterpoints. For example, water (H2O) has a melting point of 4oC and a boiling point of 100oC compared with NaCl that has a melting point of 801oC and a boiling point of 1,413oC. This is because the full charges created in ionic bonds have much stronger attractive force than the comparatively weak partial charges created in covalent molecules. thus, ionic compounds tend to form very strong crystalline lattice structures due to the repeating charges of the cation and anion components. Covalent compounds, on the otherhand, do not typically have such well-structured 3-dimensional shapes. Thus they tend to be more brittle and break more easily when in solid form, and many are found in liquid and gas phases. In addition, due to their lack of charges, they tend to be poor electrical and thermal conductors. Many are also insoluble in water due to their nonpolar nature (ie oil and water don’t mix).
Table 5.2 shows common differences between covalent and ionic compounds.
Table 5.2 Comparison of Ionic and Covalent Compounds
5.5 Naming Binary Molecular Compounds
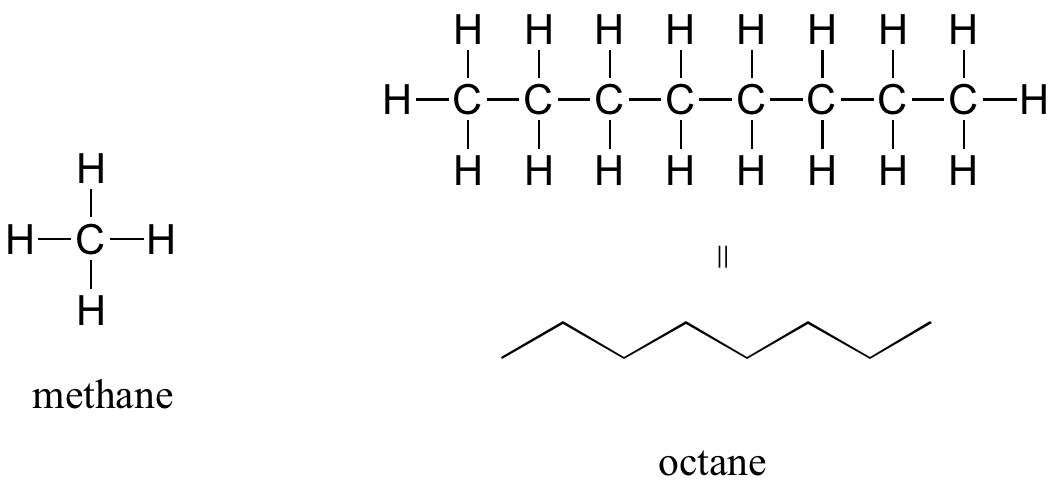
Recall that a molecular formula shows the number of atoms of each element that a molecule contains. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is H2O. A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen. The molecular formula of octane is C8H18. When writing the chemical formula the element that is the least electronegative (the element that is farther left or further down within the same family group) is written first while the more electronegative element is written second. You will be required to know how to name simple binary covalent compounds (compounds composed of two different elements)
.

Figure 5.9 Nitrogen dioxide (NO2) is a reddish-brown toxic gas that is a prominent air pollutant produced by internal combustion engines.
The elements that combine to form binary molecular compounds are both nonmetal atoms or they are a combination of a nonmetal and a metalloid. This contrasts with ionic compounds, which were formed from a metal ion and a nonmetal ion. Therefore, binary molecular compounds are different because ionic charges cannot be used to name them or to write their formulas. Another difference is that two nonmetal atoms will frequently combine with one another in a variety of ratios. Consider the elements nitrogen and oxygen. They combine to make several compounds including:
NO, NO2, and N2O
They all can’t be called nitrogen oxide. How would someone know which one you were talking about? Each of the three compounds has very different properties and reactivity. A system to distinguish between compounds such as these is necessary.
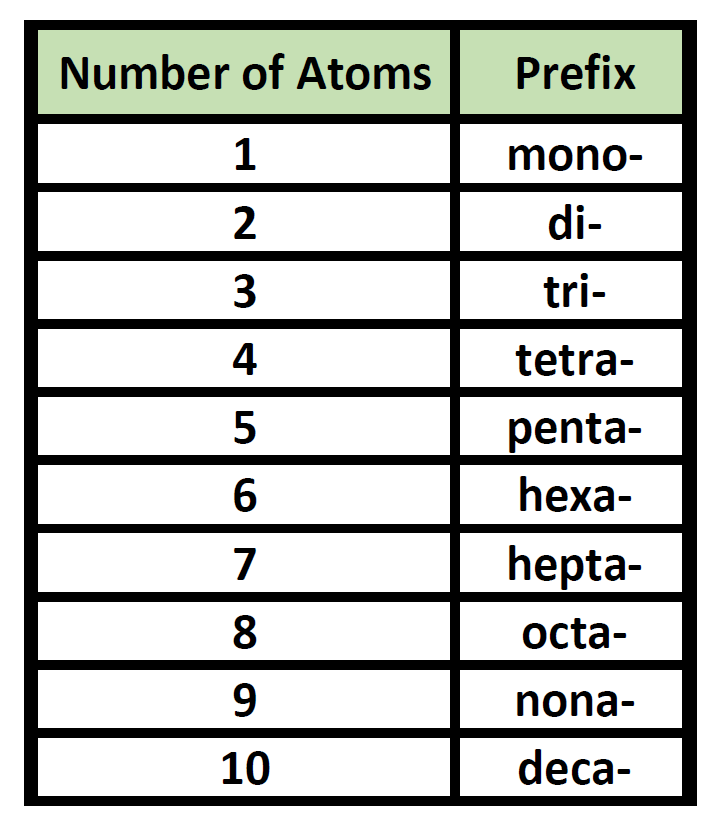
Prefixes are used in the names of binary molecular compounds to identify the number of atoms of each element. The table below shows the prefixes up to ten.
Table 5.3 Prefixes used for Nomenclature of Binary Covalent Molecules
The rules for using the prefix system of nomenclature of binary compounds can be summarized as follows.
- Generally, the less-electronegative element is written first in the formula, though there are a few exceptions. Exception 1: Carbon is always first in a formula. Exception 2: When hydrogen is participating in a covalent bond, it is typically written in the second postion (For example: hydrogen is after nitrogen in a formula such as NH3) Overall, the order of common nonmetals in binary molecular compounds is C, P, N, H, S, I, Br, Cl, O,
- When naming the first element, use the full name of the element and the appropriate prefix if there are more than one atom of that element in the formula. If there is only one atom for the first element, the term mono- is NOT used, but is implied. For example, CO is carbon monoxide, not monocarbon monoxide.
- For the second element the ending of the element’s name is typically changed to ‘-ide’ and the appropriate prefix is always used for the second element.
Note: the a or o at the end of a prefix is usually dropped from the name when the name of the element begins with a vowel. As an example, four oxygen atoms, is tetroxide instead of tetraoxide. Some examples of molecular compounds are listed in Table 5.4.
Table 5.4 Examples of Naming Covalent Molecules
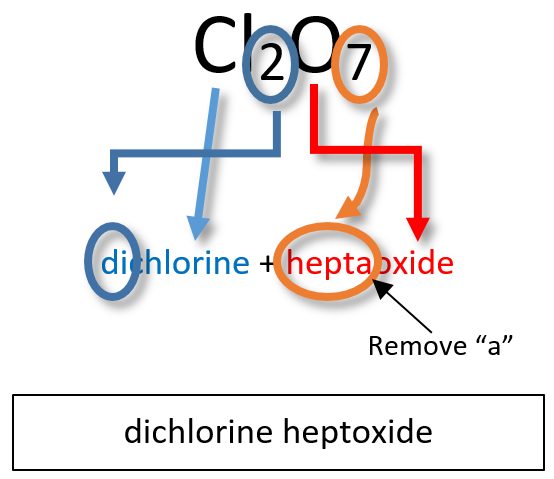
Notice that the mono- prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The S2Cl2 emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. The o of the mono- and the a of hepta- are dropped from the name when paired with oxide. For example:
(back to the top)
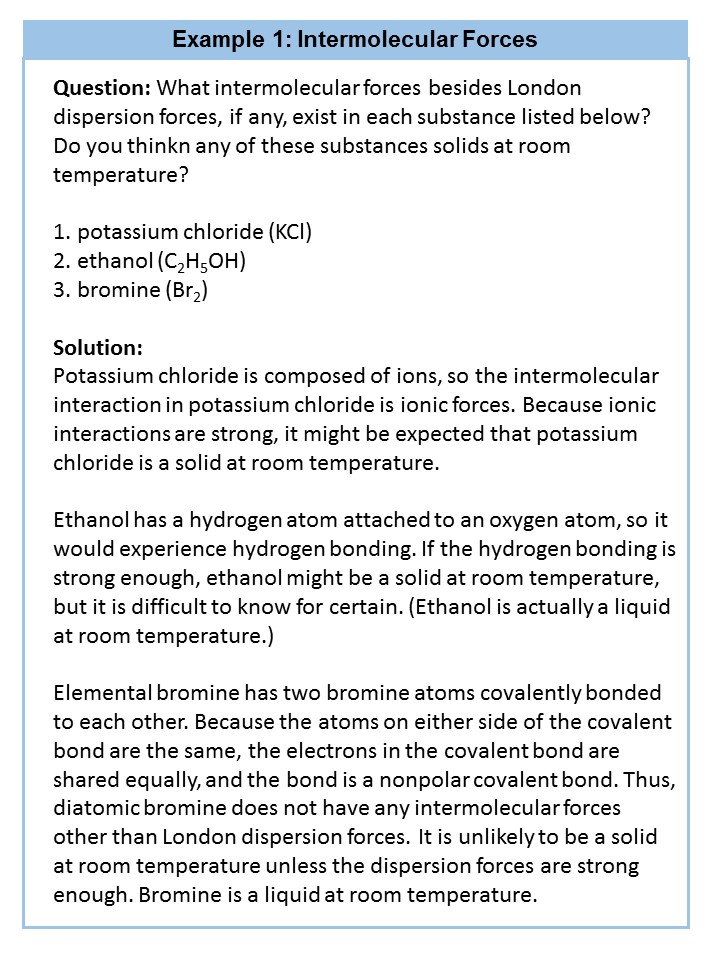
5.6 Intermolecular Forces
In addition to learning about how elements join together to form bonds, it is also very important to learn about how molecules interact with other molecules around them. This type of interaction, known as an intermolecular interaction, is important for determining broader characteristics of the molecule including reactivity and function.
Intermolecular interactions between molecules are dependent on the phase that the molecule exists. A phase is a certain form of matter that includes a specific set of physical properties. That is, the atoms, the molecules, or the ions that make up the phase do so in a consistent manner throughout the phase. As mentioned in Chapter 2, science recognizes three stable phases: the solid phase, in which individual particles can be thought of as in contact and held in place (defined volume and shape); the liquid phase, in which individual particles are in contact but moving with respect to each other (defined volume but, shape of the container); and the gas phase (no defined shape or volume), in which individual particles are separated from each other by relatively large distances. Not all substances will readily exhibit all phases on the Earth. For example, carbon dioxide does not exhibit a liquid or solid phase on Earth unless the pressure is greater than about six times normal atmospheric pressure. Other substances, especially complex organic molecules, may decompose or breakdown at higher temperatures, rather than becoming a liquid or a gas. For example, think about roasting a marshmallow. If it gets too close to the flames it will become charred and blackened, breaking down the sugar molecules inside. The sugar is not converted into the liquid or gaseous phase. Thus, water is very unique in its ability to exist on the Earth in all three phase states (solid ice – liquid water – water vapor).
Which phase a substance adopts depends on the pressure and the temperature it experiences. Of these two conditions, temperature variations are more obviously related to the phase of a substance. When it is very cold, H2O exists in the solid form as ice. When it is warmer, the liquid phase of H2O is present. At even higher temperatures, H2O boils and becomes steam (gaseous phase).
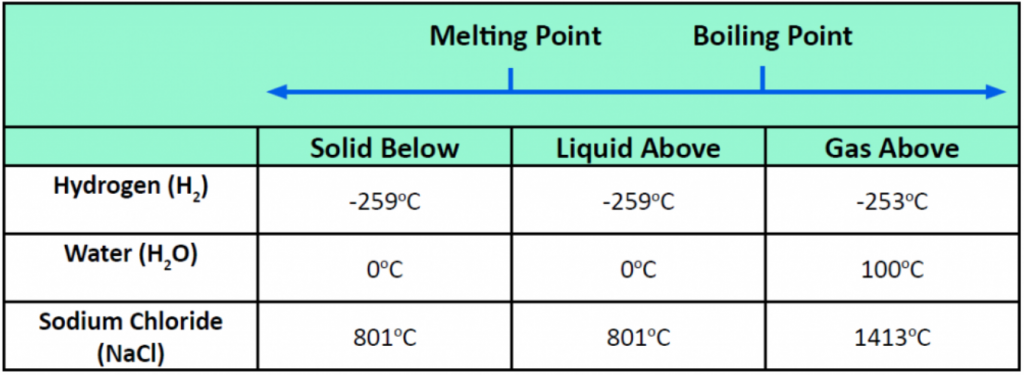
Pressure changes can also affect the presence of a particular phase (as we indicated for carbon dioxide), but its effects are less obvious most of the time. We will mostly focus on the temperature effects on phases, mentioning pressure effects only when they are important. Most chemical substances follow the same pattern of phases when going from a low temperature to a high temperature: the solid phase, then the liquid phase, and then the gas phase. However, the temperatures at which these phases are present differ for all substances and can be rather extreme. Table 5.5 shows the temperature ranges for solid, liquid, and gas phases for three substances. As you can see, there is extreme variability in the temperature ranges. Recall that the melting point of a substance is the temperature that separates a solid and a liquid. The boiling point of a substance is the temperature that separates a liquid and a gas.
Table 5.5 Each Substance has a Characteristic Melting Point and Boiling Point
What accounts for this variability? Why do some substances become liquids at very low temperatures, while others require very high temperatures before they become liquids? It all depends on the strength of the intermolecular interactions between the particles of substances. (Although ionic compounds are not composed of discrete molecules, we will still use the term intermolecular to include interactions between the ions in such compounds.) Substances that experience strong intermolecular interactions require higher temperatures to become liquids and, finally, gases. Substances that experience weak intermolecular interactions do not need much energy (as measured by temperature) to become liquids and gases and will exhibit these phases at lower temperatures.
Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. Liquids boil when the molecules have enough thermal energy to overcome the intermolecular attractive forces that hold them together, thereby forming bubbles of vapor within the liquid. Similarly, solids melt when the molecules acquire enough thermal energy to overcome the intermolecular forces that lock them into place in the solid.
Intermolecular forces are electrostatic in nature; that is, they arise from the interaction between positively and negatively charged species. Like covalent and ionic bonds, intermolecular interactions are the sum of both attractive and repulsive components. Because electrostatic interactions fall off rapidly with increasing distance between molecules, intermolecular interactions are most important for solids and liquids, where the molecules are close together. These interactions become important for gases only at very high pressures, where they are responsible for the observed deviations from the ideal gas law at high pressures.
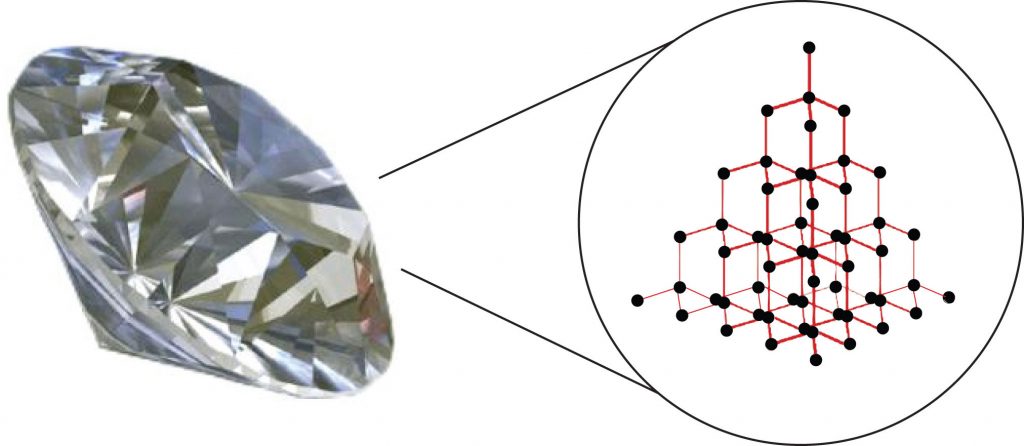
Substances with the highest melting and boiling points have covalent network bonding. This type of interaction is actually a covalent bond. In these substances, all the atoms in a sample are covalently bonded to the other atoms; in effect, the entire sample is essentially one large molecule. Many of these substances are solid over a large temperature range because it takes a lot of energy to disrupt all the covalent bonds at once. One example of a substance that shows covalent network bonding is diamond (Figure 5.10), which is a form of pure carbon. At temperatures over 3,500°C, diamond finally vaporizes into gas-phase atoms.
Figure 5.10. Diamond. Diamond, a form of pure carbon, has covalent network bonding. It takes a very high temperature—over 3,500°C—for diamond to leave the solid state.
Source: Photo © Thinkstock
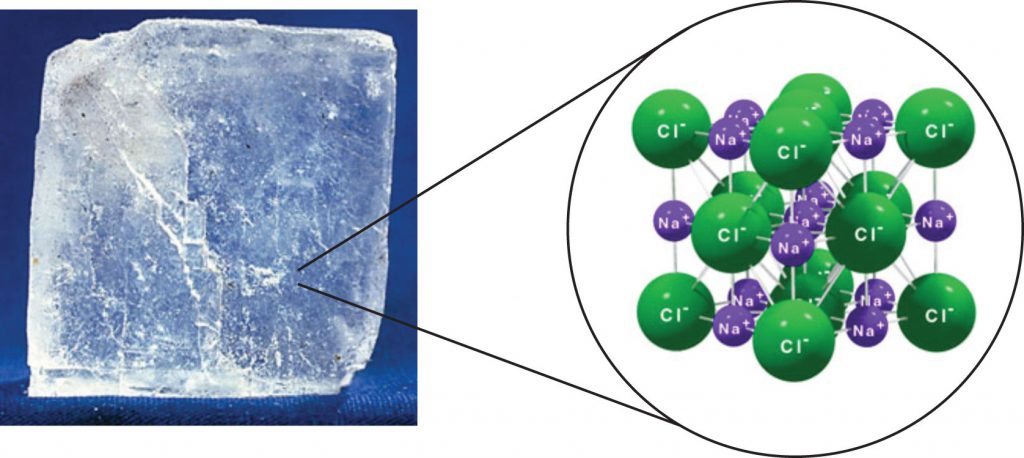
For interactions between different molecules, the strongest force between any two particles is the ionic bond, in which two ions of opposing charge are attracted to each other. Thus, ionic interactions between particles are an intermolecular interaction. Substances that contain ionic interactions are strongly held together, so these substances typically have high melting and boiling points. Sodium chloride (Figure 5.11) is an example of a substance whose particles experience ionic interactions.
Figure 5.11 Sodium Chloride. Solid NaCl is held together by ionic intermolecular forces. Source: Photo © Thinkstock
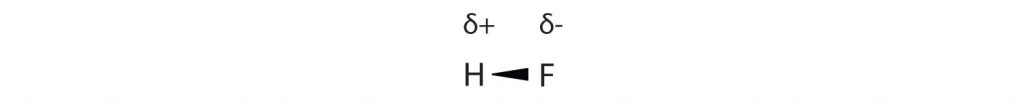
Many substances that experience covalent bonding exist as discrete molecules and do not engage in covalent network bonding. Thus, most covalently bonded molecules will also experience intermolecular forces. These intermolecular forces are weaker than those found in ionic interactions and depend on the polarity of the covalent bond. Recall that in polar covalent bonds, the electrons that are shared in a covalent bond are not shared equally between the two atoms in the bond. Typically, the atom displaying higher electronegativity attracts the electrons more strongly than the other, leading to the unequal sharing of electrons in the bond. This sets up a permanent dipole within the molecule, where one end of the molecule has a partial negative charge (δ−) and one end has a partial positive charge (δ+). This idea is illustrated in Figure 5.12, which shows a diagram of the covalent bond in hydrogen fluoride (HF).
Figure 5.12 Polar Covalent Bonds. The electrons in the HF molecule are not equally shared by the two atoms in the bond. Because the fluorine atom has nine protons in its nucleus, it attracts the negatively charged electrons in the bond more than the hydrogen atom does with its one proton in its nucleus. Thus, electrons are more strongly attracted to the fluorine atom, leading to an imbalance in the electron distribution between the atoms. The fluorine side of the bond picks up a partial overall negative charge (represented by the δ− in the diagram), while the hydrogen side of the bond has an overall partial positive charge (represented by the δ+ in the diagram). Such a bond is called a polar covalent bond.
The fluorine atom attracts the electrons in the bond more than the hydrogen atom does. The result is an unequal distribution of electrons in the bond, favoring the fluorine side of the covalent bond. Because of this unequal distribution, the fluorine side of the covalent bond actually takes on a partial negative charge (indicated by the δ− in Figure 5.12), while the hydrogen side of the bond, being electron deficient, takes on a partial positive charge (indicated by the δ+ in Figure 5.12). A covalent bond that has an unequal sharing of electrons is called a polar covalent bond. (A covalent bond that has an equal sharing of electrons, as in a covalent bond with the same atom on each side, is called a nonpolar covalent bond.) A molecule with a net unequal distribution of electrons in its covalent bonds is a polar molecule. HF is an example of a polar molecule.
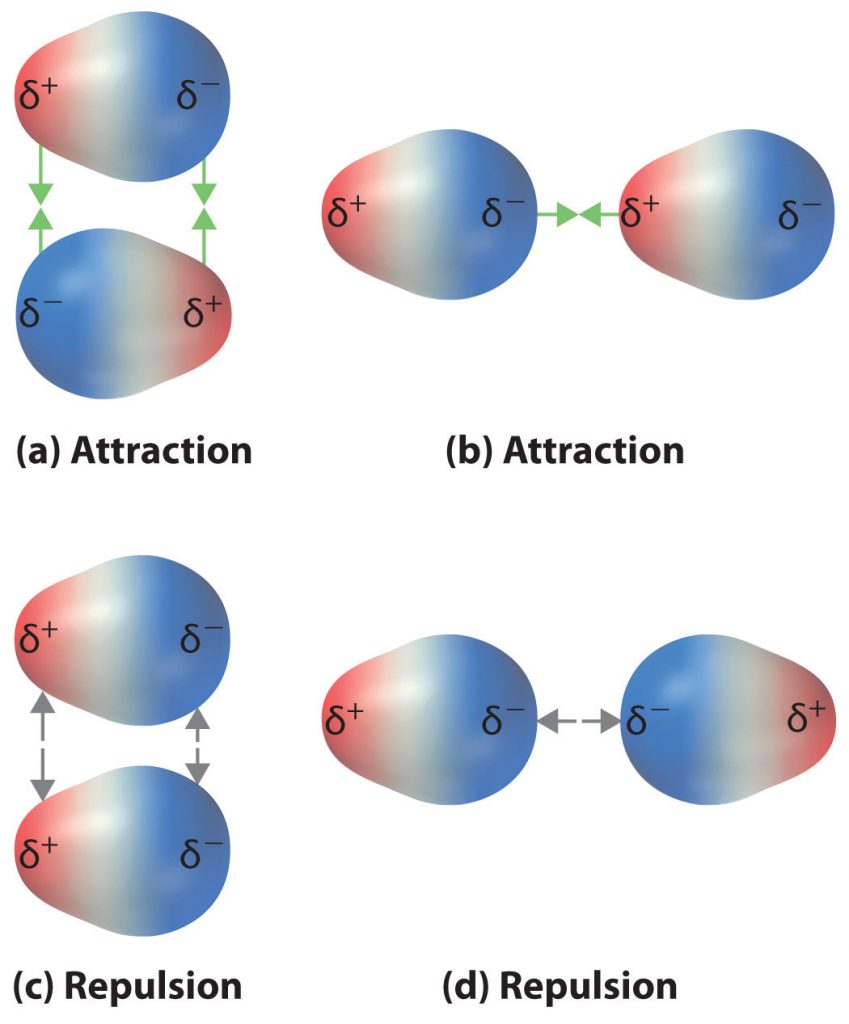
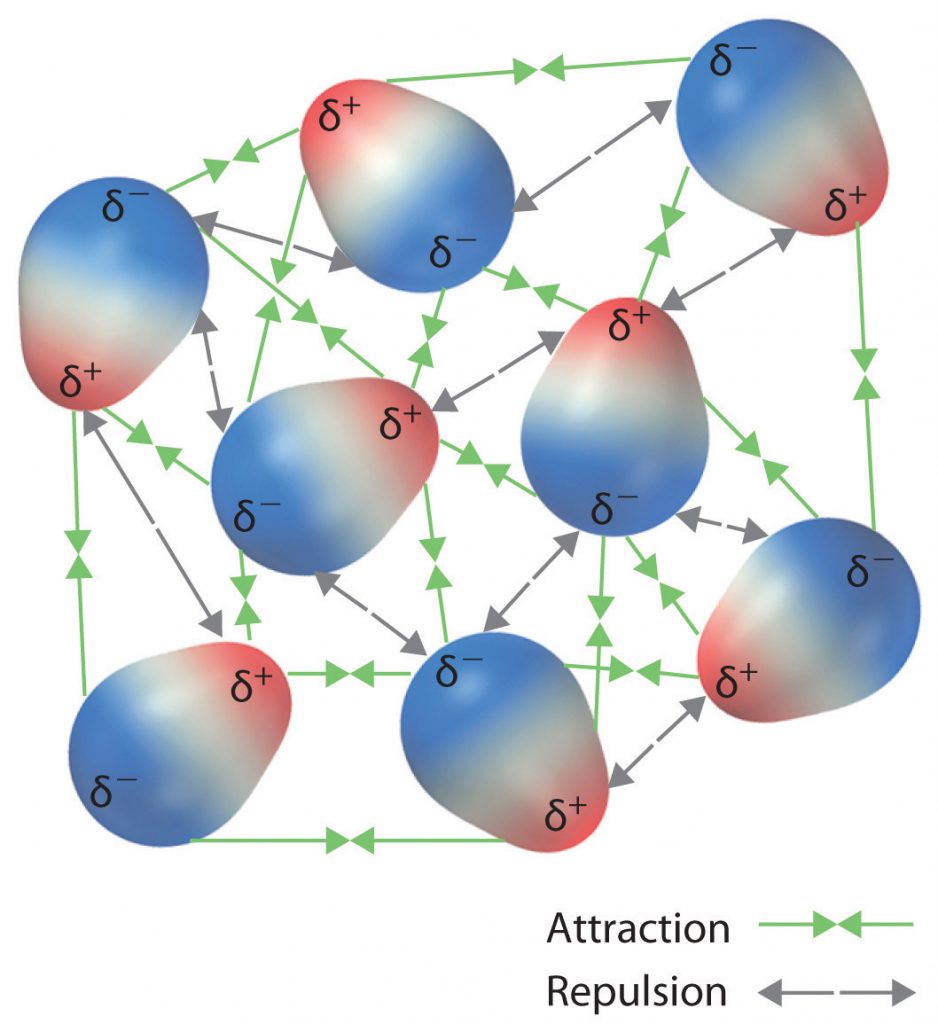
The charge separation in a polar covalent bond is not as extreme as is found in ionic compounds, but there is a related result: oppositely charged ends of different molecules will attract each other. This type of intermolecular interaction is called a dipole-dipole interaction. If the structure of a molecule is polar, then the molecule has a net dipole moment. Molecules with net dipole moments tend to align themselves so that the positive end of one dipole is near the negative end of another and vice versa, as shown in part (a) in Figure 5.13. These arrangements are more stable than arrangements in which two positive or two negative ends are adjacent (Figure 5.13, part c). Hence dipole–dipole interactions, such as those in part (b) in Figure 5.13, are attractive intermolecular interactions, whereas those in part (d) in Figure 5.13 are repulsive intermolecular interactions. Because molecules in a liquid move freely and continuously, molecules always experience both attractive and repulsive dipole–dipole interactions simultaneously, as shown in Figure 5.14. On average, however, the attractive interactions dominate.
Figure 5.13 Attractive and Repulsive Dipole-Dipole Interactions. (a and b) Molecular orientations in which the positive end of one dipole (δ+) is near the negative end of another (δ−) (and vice versa) produce attractive interactions. (c and d) Molecular orientations that juxtapose the positive or negative ends of the dipoles on adjacent molecules produce repulsive interactions.
Figure 5.14 Both Attractive and Repulsive Dipole-Dipole Interactions Occur in a Liquid Sample with Many Molecules.
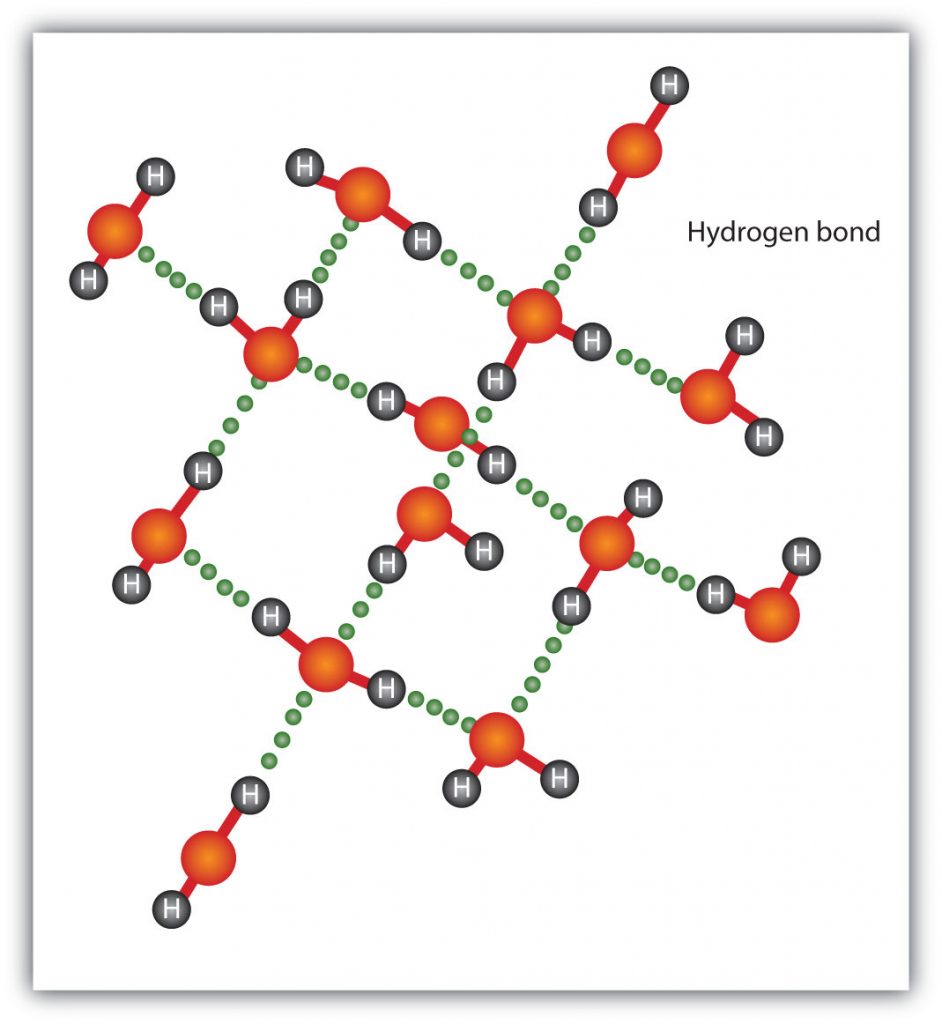
The H–F, O–H, and N–H bonds are strongly polar; In molecules that have these bonds, particularly strong dipole-dipole interactions (as strong as 10% of a true covalent bond) can occur. Because of this strong interaction, hydrogen bonding is used to describe this dipole-dipole interaction. The physical properties of water, which has two O–H bonds, are strongly affected by the presence of hydrogen bonding between water molecules. Figure 5.15 shows how molecules experiencing hydrogen bonding can interact in water.
Figure 5.15 Hydrogen Bonding between Water Molecules. The presence of hydrogen bonding in molecules like water can have a large impact on the physical properties of a substance.
Finally, there are forces between all molecules that are caused by electrons being in different places in a molecule at any one time, which sets up a temporary separation of charge that disappears almost as soon as it appears and sets up a momentary ‘induced dipole’. These are very weak intermolecular interactions and are called London dispersion forces. Since electrons naturally orbit the nucleus of the atom, there are momentary dipoles that are present in the atom as the electrons are shifting from one side to the other. If other atoms are in close proximity, the electrons of the other atoms will orbit in concert with the neighboring atom, i.e. the electrons of one atom are repulsive to the electrons of the neighboring atoms, such that when they are close to the neighboring atom, the neighboring electrons will shift away to the other side of the atom. Thus, the electon movements between atoms of different molecules will synchronize and orbit in a pattern that maximizes the distance between electrons of a neighboring atom. Note that all substances experience London dispersion forces. However, these are the only intermolecular forces that nonpolar covalent compounds experience. Nonpolar covalent molecules tend to be soft in the solid phase and have relatively low melting points. Butter fat would be a good example of a nonpolar covalent compound.
Because London dispersion forces are caused by the instantaneous distribution of electrons in a molecule, larger molecules with a large number of electrons can experience higher levels of London dispersion forces. Examples include waxes, which are long hydrocarbon chains that are solids at room temperature because the molecules have so many electrons. The resulting dispersion forces between these molecules make them assume the solid phase at normal temperatures.
Video Tutorial on London Dispersion Forces By Bozeman Science.
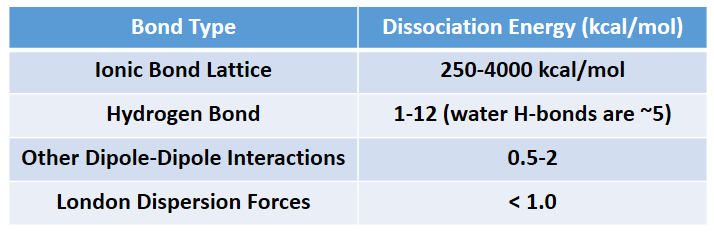
The phase that a substance adopts depends on the type and magnitude of the intermolecular interactions the particles of a substance experience. If the intermolecular interactions are relatively strong, then a large amount of energy—in terms of temperature—is necessary for a substance to change phases. If the intermolecular interactions are weak, a low temperature is all that is necessary to move a substance out of the solid phase. Overall, Ionic interactions are the strongest intermolecular forces followed by hydrogen bonding, other dipole-dipole interactions, and lastly, induced dipoles (London dispersion forces). Intermolecular force strength is indicated in Table 5.6.
Table 5.6 Strength of Intermolecular Forces
5.7 Recognizing and Drawing Organic Molecules
As noted in section 5.1, organic compounds are compounds that contain carbon and hydrogen. Another notable feature of organic molecules is that they are quite complex and contain many atoms of carbon and hydrogen as well as other heteroatoms (atoms other than carbon or hydrogen) that are held together through covalent bonding. The most common heteroatoms that will be found in organic molecules include oxygen, nitrogen, sulfur, phosphorous, and occasionally halides (Cl, Br, and I). Since they can be quite complex, it is useful to discuss the many different ways that organic compounds can be represented/drawn.
Molecular Formula
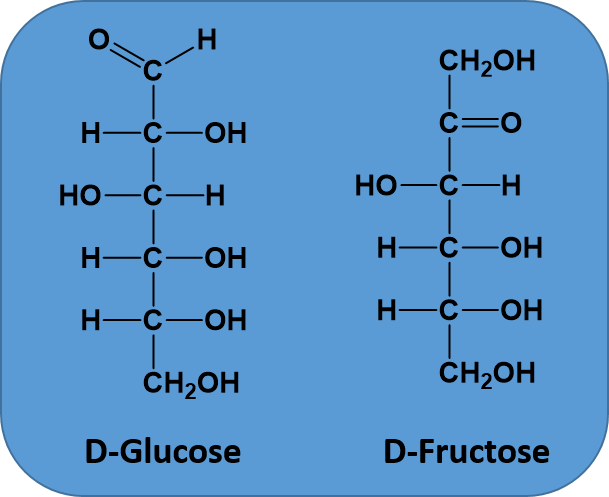
A molecular formula is the simplest way to represent a compound by counting up all of the different types of atoms and listing them in order. For example, the sugar glucose, contains 6 carbons, 12 hydrogens, and 6 oxygens. The molecular formula would then be written as C6H12O6. By convention, carbon is listed first, hydrogen second, followed by oxygen, nitrogen, sulfur, phosphorus, and finally any halogens. However, for organic chemistry, molecular formulae don’t provide much information. They simply provide the numbers of each type of atom present in the molecule, but they tell you nothing about the way the atoms are joined together in space. For example, two molecules might have the same molecular formula, but a different arrangement of the atoms bonded in space, as is the case for the two sugars glucose and fructose. Both sugars have the molecular formula of C6H12O6. However, you can see from Figure 5.16, that they are different molecules with different properties, because the atoms are linked together in a different order. Molecules that share the same molecular formula but have their atoms bonded in a different order are called isomers. Due to the complexity of isomer structures, molecular formulae not as often used in organic chemistry, because they do not give useful information about the bonding order within the molecule.
Figure 5.16 Structural Isomers. When molecules, such as D-Glucose and D-Fructose, share the same molecular formula, but have a different atomic bonding order they are called structural isomers.
Structural formulae and 3-dimensional models
A structural formula shows how the various atoms are bonded, and can be more useful that only writing the molecular formula for a compound. There are various ways of drawing structural formulae and you will need to be familiar with all of them. They include the displayed formula, condensed formulas, and line structures.
Displayed formulae
A displayed formula shows all the bonds in the molecule as individual lines with each atom written at the end of each line using its elemental abbreviation from the periodic table. The structures of C6H12O6, above, are all written in displayed formulae. You need to remember that each line represents a pair of shared electrons. For example, figure 5.17 below depicts the displayed formula of methane next to the three-dimensional representations.
Figure 5.17: Three different representations of CH4 On the left is the ball and stick model, in the center is the displayed formula, and to the right is the space-filling model.
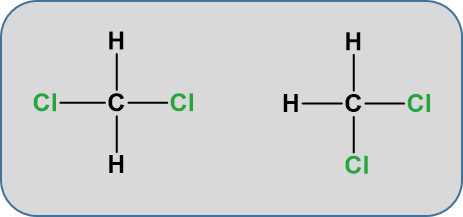
Notice that the displayed formula of methane does not represent the 3-D shape of the molecule shown in the space-filling diagram on the right. Methane isn’t flat with 90° bond angles. This mismatch between what you draw and what the molecule actually looks like can lead to problems if you aren’t careful. Thus, for organic chemistry, it is important to begin thinking about the structures in their 3-D form. The more you practice, the more you will be able to visualize and turn the molecule around in your head. For example, consider the simple molecule with the molecular formula CH2Cl2. You might think that there were two different ways of arranging these atoms if you drew a displayed formula.
But these two structures are actually exactly the same. When atoms are sharing electrons with other atoms, they tend to take on three dimensional spatial relationships that keep the electrons in shared pairs as far away as possible from other electrons in shared pairs. This tendency is called valence shell electron pair repulsion theory or VESPR. Due to this tendency, since carbon forms four bonds, it will take on a tetrahedral confirmation where each bond angle is 109o. The molecule is not flat, in the plane of the paper. Play the video below to see how they appear as rotating ball and stick models.
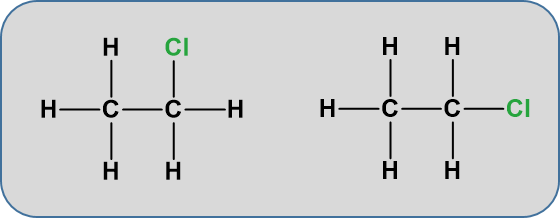
One structure is in reality a simple rotation of the other one. Consider a slightly more complicated molecule, C2H5Cl. The displayed formula could be written as either of these:
But, again these are exactly the same. Look at the models below.
As you continue to practice drawing out structural formulae, you will become better at recognizing and distinguishing between isomers that are truly different from one another, and versions of the same molecule written drawn from different 3-dimensional perspectives.
Condensed formulae
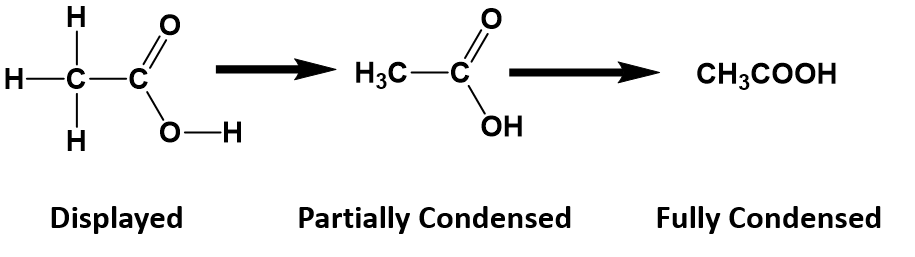
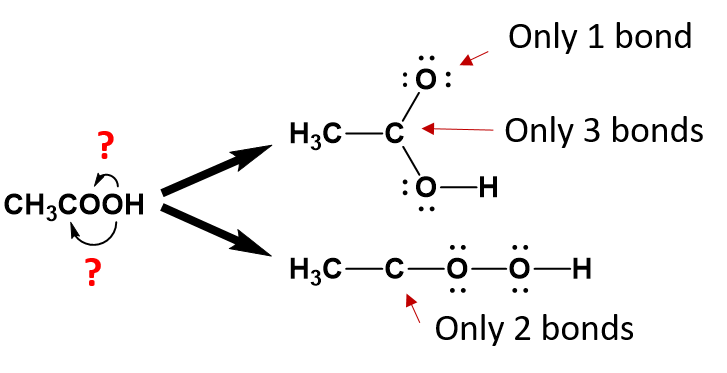
For anything other than the most simple molecules, drawing a fully displayed formula can be cumbersome and take up too much space – especially all the carbon-hydrogen bonds. You can simplify – or condense – the formula by writing, for example, CH3 or CH2 instead of showing all the C-H bonds. For example, ethanoic (C2H4O2) acid can be shown in a fully displayed form, a partially condensed form and a fully condensed form.
Notice that the partially condensed structure still provides a very clear picture of where each of the atoms is bonded in space. However, with the fully condensed structure, it can be challenging to accurately see the bonding patterns. The fully condensed form does contain more information about bonding order than the molecular formula, such that the atoms that are directly bonded to a neighboring atom are placed adjacent to that atom in the condensed form, rather than a simple tallying of the total atom species as in the molecular formula.
By working backwards, we can use the condensed structure of ethanoic acid as an example to recreate the partially condensed structure. When looking at the first carbon position, it is apparent that there are three hydrogens and one carbon bound to the first carbon:
Note that this satisfies the octet rule for the first carbon (four bonds to other atoms). The three hydrogens are also complete with their single bonds to the first carbon. The second carbon has now been assigned one bond to the first carbon. We need to assign the remaining three bonds.
From the condensed formula, it is clear that the first oxygen is attached to the second carbon, however, after that, we become unsure about the position of the second oxygen.
We can clearly deduce that the last hydrogen atom is bound to the second oxygen, as it is placed in that position. However, because oxygen can form two bonds, we can’t be sure based on the condensed structure alone, that the second oxygen is bound to the second carbon or to the first oxygen.
When you are unsure of which atom is bonded to which, it is best to draw out the potential structures and evaluate them for their potential correctness.
From the analysis of the potential structures above, it is clear that neither structure satisfies the octet rule for one or more atoms within the molecule as currently written. However, the lower structure is less satisfactory than the upper structure, as the second carbon is missing 2 covalent bonds while all of the other atoms have satisfied the octet bonding requirements. In the upper diagram, both the second carbon and the first oxygen atom are lacking one bond. This structure can easily satisfy the octet rule by placing a double bond between carbon 2 and oxygen 1 within the molecule. Whereas, a solution for the missing two carbon bonds for the second carbon in the lower structure is not easily remedied. Thus, the upper structure is a more probable structure than the lower structure with the addition of the double bond between the carbon and the oxygen.
While condensed structures are easier to write than displayed or partially condensed structures they can prove to be a little more challenging to determine the three dimensional bonding pattern of the atoms.
Line or Skeletal Formulae
In a line or skeletal formula, all the hydrogen atoms are not shown and all the carbons are not labeled but rather are indicated at the end or bend in every line, leaving just a carbon skeleton with functional groups attached to it. Any heteroatoms (any other atom than carbon or hydrogen) and hydrogens attached to heteroatoms are shown in condensed form. For example, the displayed structure, partially condensed structure and the line formula for 2-butanol (C4H10O) look like this:
In a line or skeletal diagram, the following assumptions can be made:
- there is a carbon atom at each line junction and at the end of each line.
- there are enough hydrogen atoms attached to each carbon to make the total number of bonds on that carbon equal to 4.
- all heteroatoms (and hydrogens attached to heteroatoms) are shown in condensed format on the skeletal structure.
Within organic chemistry and biochemistry, scientists tend to use a combination of these different formats to represent chemical structures. It is important to become familiar with drawing and interpreting all the different possible representations.
(back to the top)
How to draw structural formulae in 3-dimensions
There are occasions when it is important to be able to show the precise 3-D arrangement in parts of some molecules when using a structural representation. To do this, the bonds are shown using conventional symbols:
For example, you might want to show the 3-D arrangement of the groups around the carbon which has the -OH group in 2-butanol.
Drawing abbreviated organic structures
Often when drawing organic structures, chemists find it convenient to use the letter ‘R’ to designate part of a molecule outside of the region of interest. If we just want to refer in general to a functional group without drawing a specific molecule, for example, we can use ‘R groups’ to focus attention on the group of interest:

The R group is a convenient way to abbreviate the structures of large biological molecules, especially when we are interested in something that is occurring specifically at one location on the molecule. For example, in chapter 15 when we look at biochemical oxidation-reduction reactions involving the flavin molecule, we will abbreviate a large part of the flavin structure (ie. R = FAD) which does not change at all in the reactions of interest:
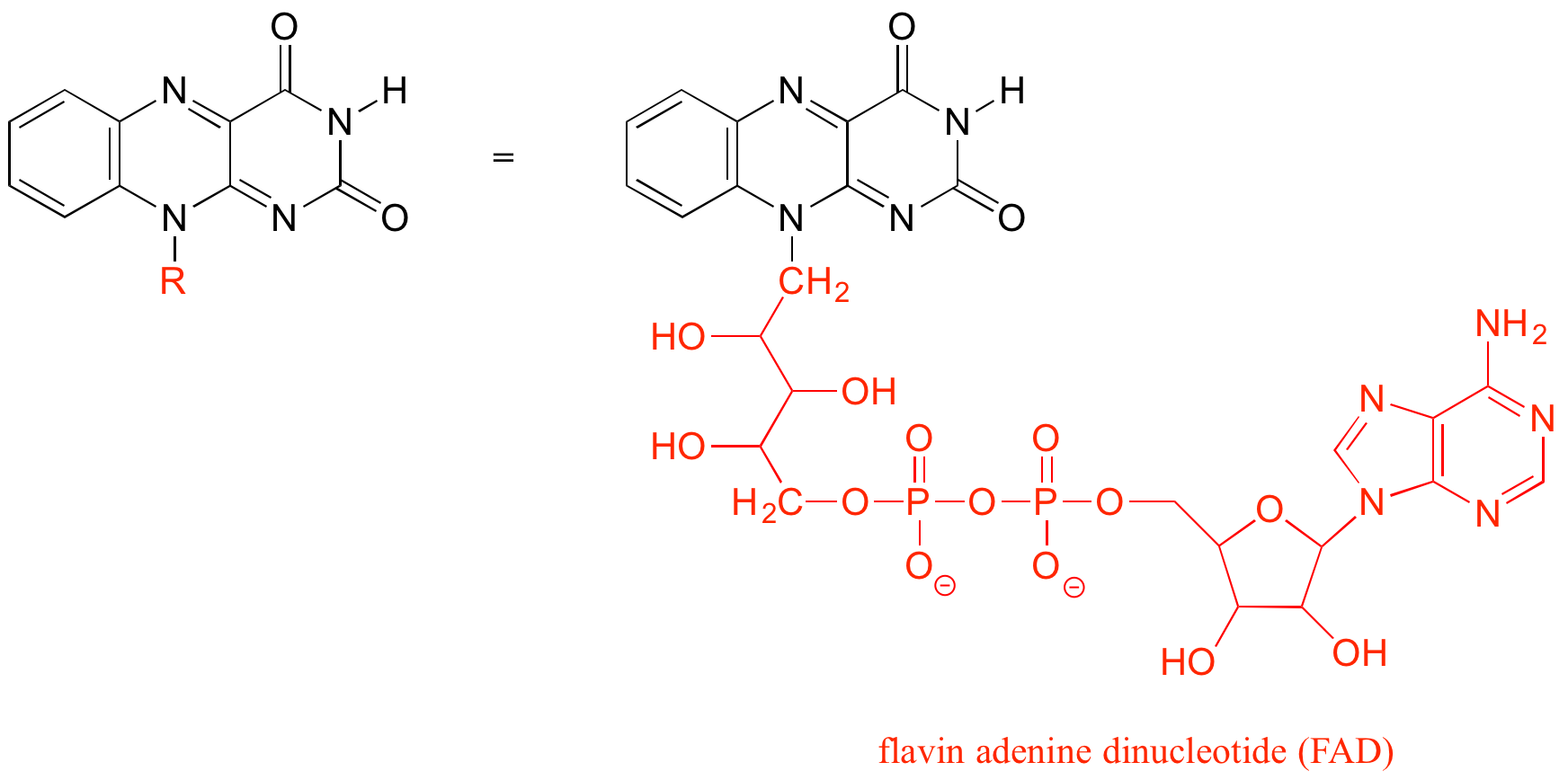
As an alternative, we can use a ‘break’ symbol to indicate that we are looking at a small piece or section of a larger molecule. This is used commonly in the context of drawing groups on large polymers such as proteins or DNA.

Finally, R groups can be used to concisely illustrate a series of related compounds, such as the family of penicillin-based antibiotics.
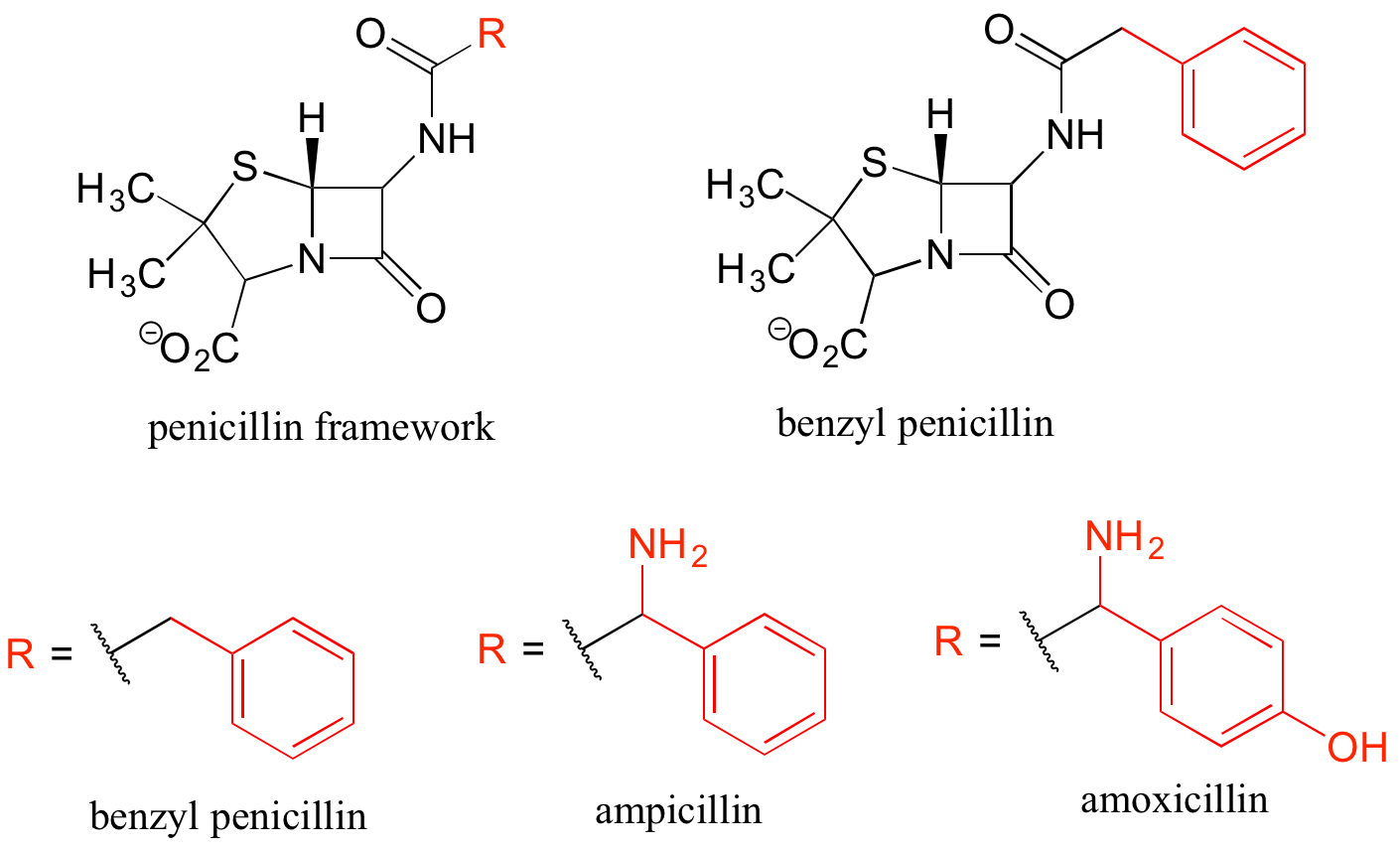
Using abbreviations appropriately is a very important skill to develop when studying organic chemistry in a biological context, because although many biomolecules are very large and complex (and take forever to draw!), usually we are focusing on just one small part of the molecule where a change is taking place.
As a rule, you should never abbreviate any atom involved in a bond-breaking or bond-forming event that is being illustrated: only abbreviate that part of the molecule which is not involved in the reaction of interest.
For example, carbon #2 in the reactant/product below most definitely is involved in bonding changes, and therefore should not be included in the ‘R’ group.
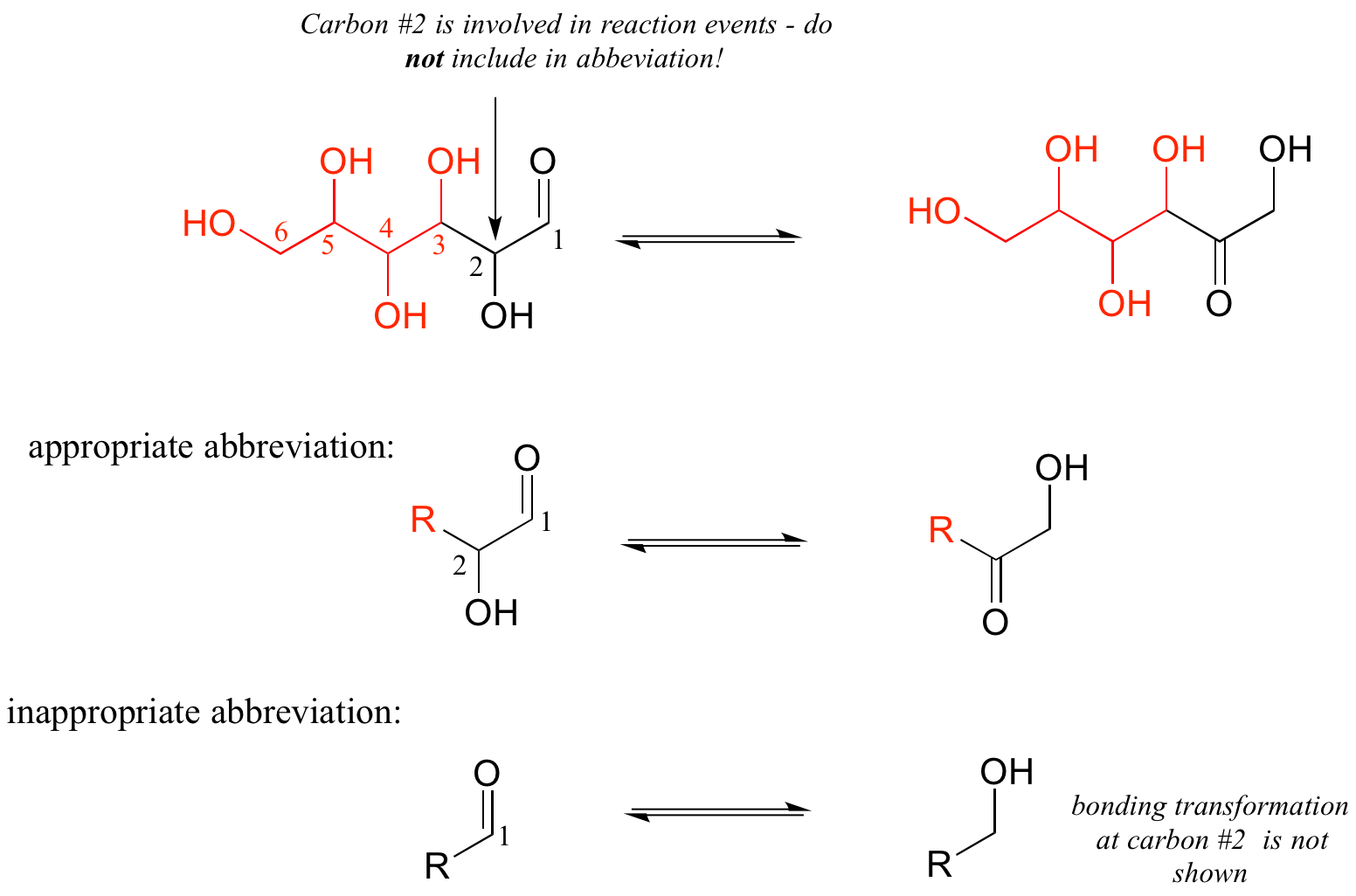
If you are unsure whether to draw out part of a structure or abbreviate it, the safest thing to do is to draw it out.
(back to the top)
5.8 Stereoisomers, Enantiomers, and Chirality
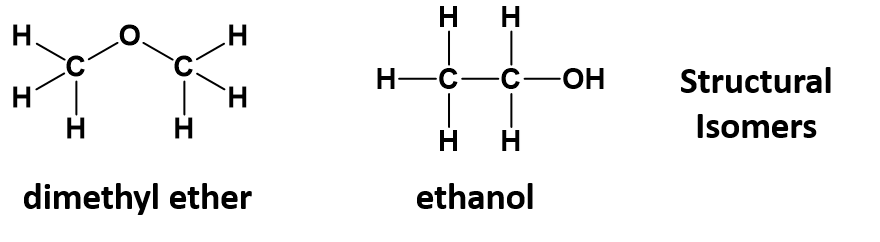
As seen in section 5.7, organic chemistry involves infinitely varied structures arising from how the atoms are assembled in 3-dimensional space. Providing only the molecular formula of a compound is often insufficient for defining the compound as many molecular formulas have numerous structural isomers. For example, the molecular formula C2H6O, a molecule of only 9 atoms, can refer to dimethyl ether or ethanol, depending on whether the oxygen is in the middle of or at the end of the carbon chain.
Remember that structural isomers have the same molecular formula, but the order that the atoms are linked together is different, leading to different physical and chemical properties. For example, ethanol is liquid at room temperature, whereas diethyl ether is a gas.
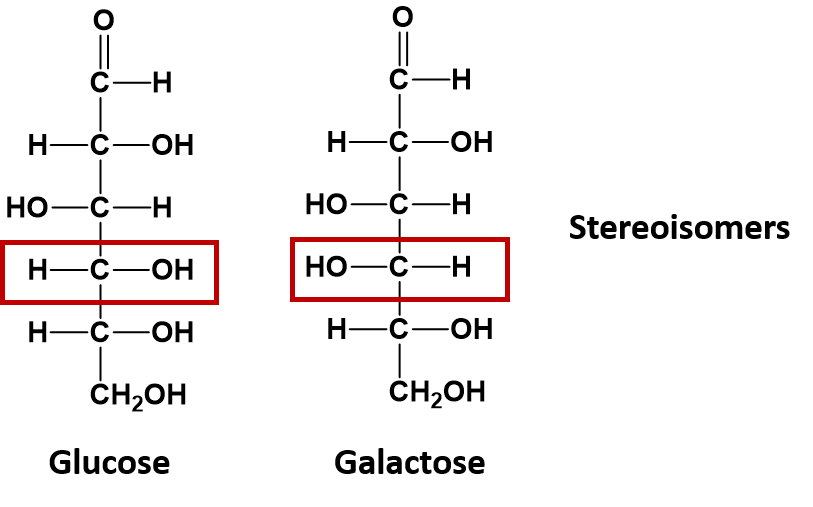
If the atoms of compounds with the same molecular formula are linked together in the same order, but their 3-dimensional arrangement in space differs, they are considered to be a special type of isomer called a stereoisomer. The sugar molecules glucose and galactose are stereoisomers. They differ in the spatial position of a single -OH group as indicated in the diagram below:
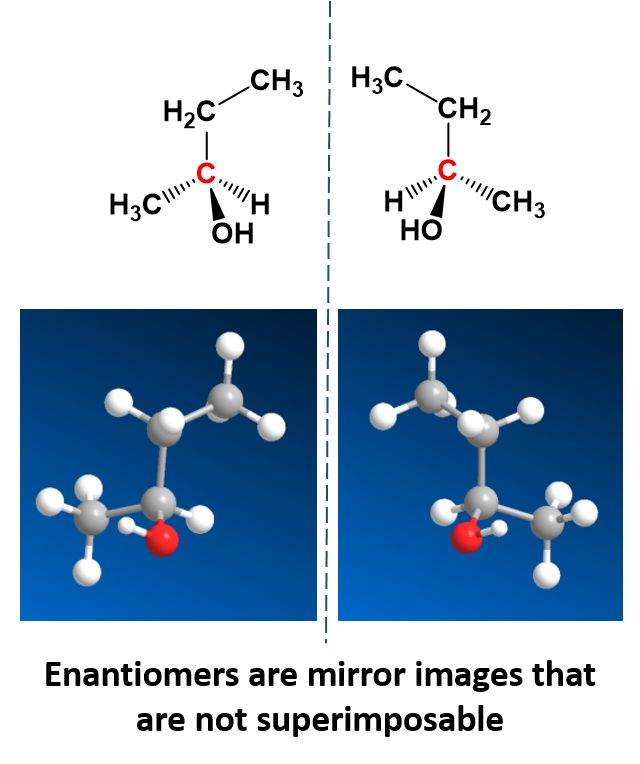
There is a special kind of stereoisomers, called enantiomers, that are mirror images of each other, but are not superimposable. This means that no matter how you turn them in space that you can never put them on top of one another and recover the same compound. One example is 2-butanol which can be drawn as a pair of enantiomers (Fig. 5.18).
Figure 5.18: The enantiomers of 2-butanol. The enantiomers are shown in the 3-D structural formula displayed in the top diagram and the ball and stick model in the lower diagram.
Chirality
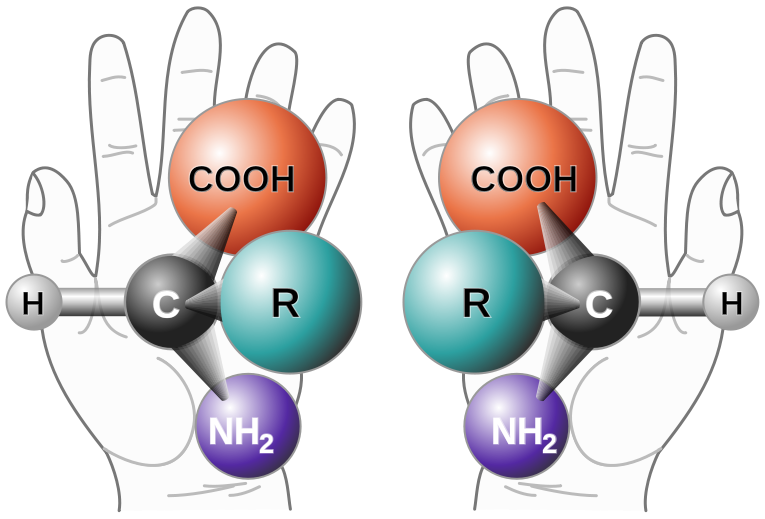
Enantiomers are said to have the property of chirality. Chirality is the term that is given to objects that are mirror images but are not superimposable. The term ‘chiral’ is derived from the Greek word for ‘handedness’ – ie. right-handedness or left-handedness. Your hands are chiral: your right hand is a mirror image of your left hand, but if you place one hand on top of the other, both palms down, you see that they are not superimposable. (Fig 5.19). Thus chiral objects are mirror images of one another, but cannot be superimposed on top of one another. Carbon becomes chiral when it has four different substituents attached to it. You will notice in the example above that the central carbon has four different groups attached to it: an -OH group, an -H, a -CH3, and a -CH2CH3 group.
Figure 5.19: The Nature of Chirality. Carbon becomes chiral when it has four different substituents bonded to it. Any way you rotate the molecule on the left, you cannot superimpose it onto the molecule on the right.
Source: Chirality with hands.jpg: Unknown derivative work: — πϵρήλιο ℗ – Chirality with hands.jpg
Stereoisomers that are not enantiomers, such as glucose and galactose shown above, do have chiral centers and are not superimposable, but they are not mirror images of one another. Only stereoisomers that are also mirror images and not superimposable are termed enantiomers. Enantiomers are very hard to separate from one another. They are nearly identical in their physical and chemical properties. They have the same molecular weight, the same polarity, the same melting and boiling points, etc. In fact, enantiomers are so alike that they even share the same name! In Figure 5.14 the two enantiomers of 2-butanol are shown. Both of the molecules are 2-butanol. But they are not exactly the same molecule, in the same way that your left shoe is not exactly the same as your right. They are non-superimposable mirror images of each other. How do we communicate this difference?
One small difference between enantiomers is the direction that polarized light will rotate when it hits the molecule. One enantiomer will rotate light in the clockwise direction, while the other will rotate it in the counterclockwise direction. The clockwise version is termed ‘D’ for dextrorotary (or right-handed) and the counterclockwise version is termed ‘L’ for levorotary (or left-handed). However, light rotation cannot be used in a predictive way to determine the absolute stereo-configuration of a molecule (i.e. you cannot tell which enantiomer is going to rotate the light to the right or to the left until you actually do the experiment).
Thus, another system is needed to describe the absolute configuration. The Cahn-Ingold-Prelog (CIP) priority system was designed to determine the absolute stereo-configuration of enantiomers as either sinister (S) or rectus (R). In this system, the groups that are attached to the chiral carbon are given priority based on their atomic number (Z). Atoms with higher atomic number (more protons) are given higher priority (i.e. S > P > O > N > C > H).
For determining the stereochemistry, place the lowest priority group away from you, so that the other three groups are held are facing you. Assign priority to the remaining groups.
The rules for this system of stereochemical nomenclature are, on the surface, fairly simple. We’ll use the simple 3-carbon sugar glyceraldehyde as our first example. Try making a model of the stereoisomer of glyceraldehyde shown below. If you don’t have a chemistry modeling kit, an easy alternative is to use toothpicks and gumdrops. Be sure that you are making the correct enantiomer!
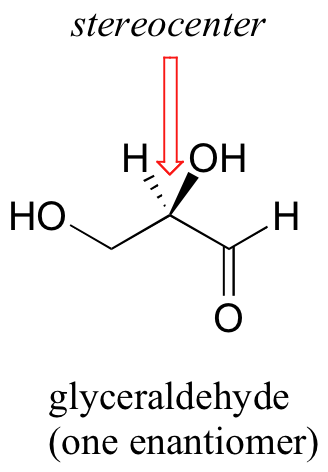
The first thing that we must do is to assign a priority to each of the four substituents bound to the chiral carbon. In this nomenclature system, the priorities are based on atomic number, with higher atomic numbers having a higher priority. We first look at the atoms that are directly bonded to the chiral carbon: these are H, O (in the hydroxyl), C (in the aldehyde), and C (in the CH2OH group).
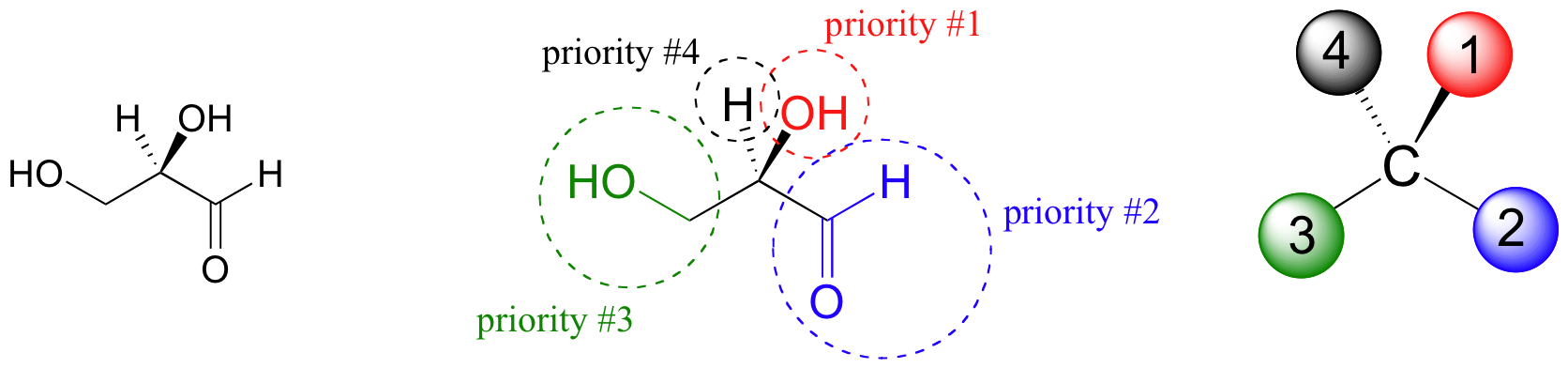
Two priorities are easy: hydrogen, with an atomic number of 1, is the lowest (#4) priority, and the hydroxyl oxygen, with atomic number 8, is priority #1. Carbon has an atomic number of 6. Which of the two ‘C’ groups is priority #2, the aldehyde (CHO) or the alcohol (CH2OH)? To determine this, we move one more bond away from the stereocenter: for the aldehyde we have a double bond to an oxygen, while on the CH2OH group we have a single bond to an oxygen. If the atom is the same, double bonds have a higher priority than single bonds. Therefore, the aldehyde group is assigned #2 priority and the CH2OH group the #3 priority. With our priorities assigned, we next make sure that the #4 priority group (the hydrogen) is pointed back away from ourselves, into the plane of the page (it is already).

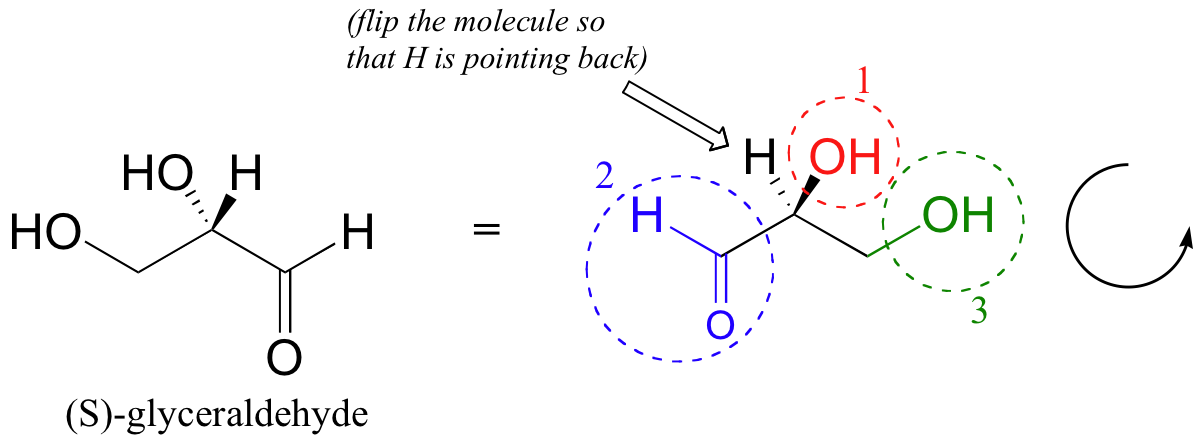
Then, we trace a circle defined by the #1, #2, and #3 priority groups, in increasing order. For our glyceraldehyde example, this circle is clockwise, which tells us that this carbon has the ‘R’ configuration, and that this molecule is (R)-glyceraldehyde. For (S)-glyceraldehyde, the circle described by the #1, #2, and #3 priority groups is counter-clockwise (but first, we must flip the molecule over so that the H is pointing into the plane of the page).
In the case of 2-butanol (Fig 5.20), the first priority and the fourth priority are easy to assign. The -OH is first priority and the -H is fourth priority. How do you assign 2nd and 3rd priority, since both of those atoms are carbon? If the priority is the same for an attached atom, you need to look out to the next level and evaluate priority there. For 2-butanol, one group is -CH3 and one group is -CH2CH3. In the first situation, if we look out to the next level, this carbon is bound to three other hydrogen atoms (all very low priority). In the second situation, the carbon is bound to two hydrogens and one carbon. Since C has a higher priority than H, the -CH2CH3 group will have higher priority over the -CH3 group. Once all of the groups have been assigned priority, you can determine which direction the priority is moving. If it is in the clockwise direction, the molecule is given the ‘R’ designation. Priority moving in the counterclockwise direction is given the ‘S’ designation. In our example, the 2-butanol on the left shows priority moving in the counterclockwise direction giving the S-enantiomer. The molecule on the right shows the R-enantiomer with priority moving in the clockwise direction.
Figure 5.20: Stereochemistry of 2-butanol. The CIP priority system can be used to determine the absolute stereo-conformation of enantiomers
Thalidomide – A Story of Unintended Consequences
Interestingly, enantiomers have the same physical properties and exactly the same chemical properties, except when reacting with other chiral molecules. Thus, chiral molecules have potentially drastic differences in physiology and medicine. For example, in the 1960’s, a drug called thalidomide was widely prescribed in Western Europe to alleviate morning sickness in pregnant women.
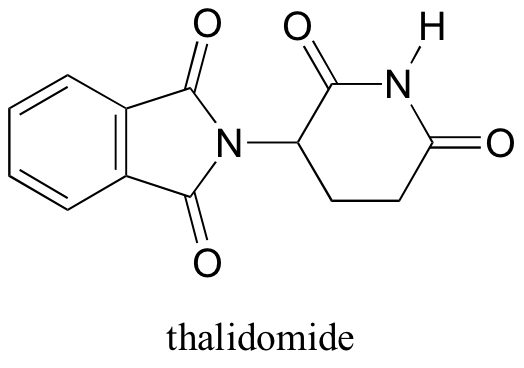
Thalidomide had previously been used in other countries as an antidepressant, and was believed to be safe and effective. It was not long, however, before doctors realized that something had gone horribly wrong: many babies born to women who had taken thalidomide during pregnancy suffered from severe birth defects.
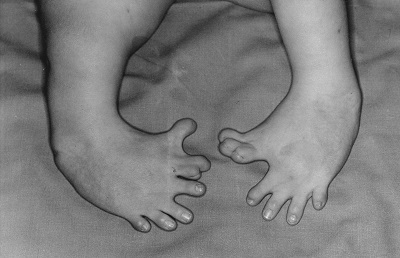 Figure 5.21: Baby born to a mother who had taken thalidomide while pregnant. (OtisArchives3, CC BY 2.0)
Figure 5.21: Baby born to a mother who had taken thalidomide while pregnant. (OtisArchives3, CC BY 2.0)
Researchers later realized the that problem lay in the fact that thalidomide was being provided as a mixture of two different isomeric forms, called a racemic mixture.
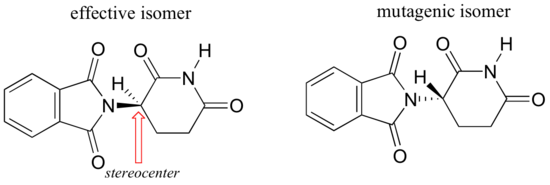
One of the isomers is an effective medication, while the other caused the side effects. Both isomeric forms have the same molecular formula and the same atom-to-atom connectivity, so they are not merely structural isomers. Where they differ is in the arrangement in three-dimensional space about one tetrahedral chiral carbon. Thus, these two forms of thalidomide are enantiomers.
Note that the carbon in question has four different substituents (two of these just happen to be connected by a ring structure). Tetrahedral carbons with four different substituent groups are called stereocenters.
Looking at the structures of what we are referring to as the two isomers of thalidomide, you may not be entirely convinced that they are actually two different molecules. In order to convince ourselves that they are indeed different, let’s create a generalized picture of a tetrahedral carbon stereocenter, with the four substituents designated R1-R4. The two stereoisomers of our simplified model look like this:
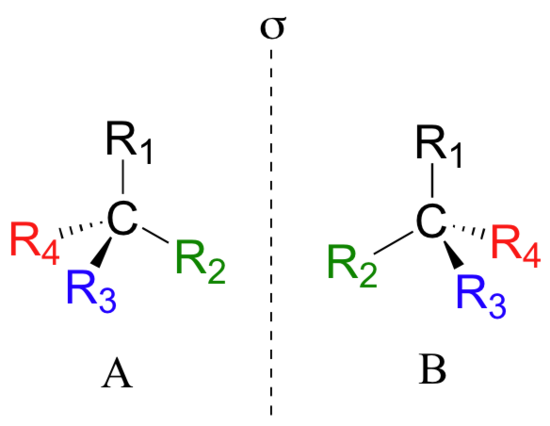
If you look carefully at the figure above, you will notice that molecule A and molecule B are mirror images of each other (the line labeled ‘σ’ represents a mirror plane). Furthermore, they are not superimposable: if we pick up molecule A, flip it around, and place it next to molecule B, we see that the two structures cannot be superimposed on each other. They are two different molecules!
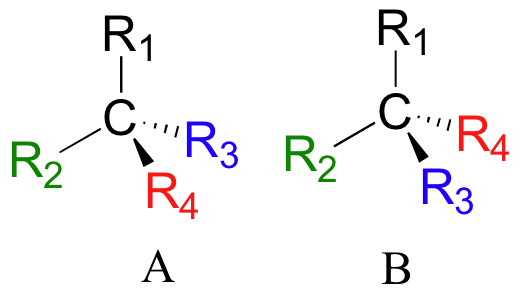
If you make models of the two stereoisomers of thalidomide and do the same thing, you will see that they too are mirror images, and cannot be superimposed (it will help to look at a color version of the figure below).
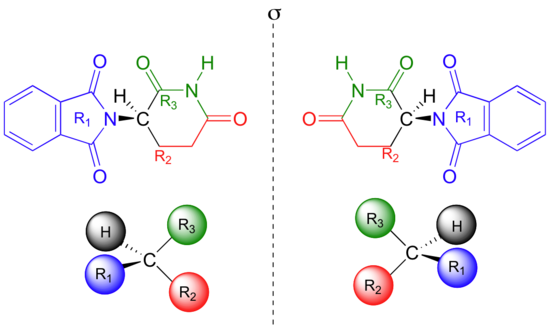
Thalidomide is a chiral molecule.
Here are some more examples of chiral molecules that exist as pairs of enantiomers. In each of these examples, there is a single stereocenter, indicated with an arrow. (Many molecules have more than one stereocenter, but we will get to that that a little later!)
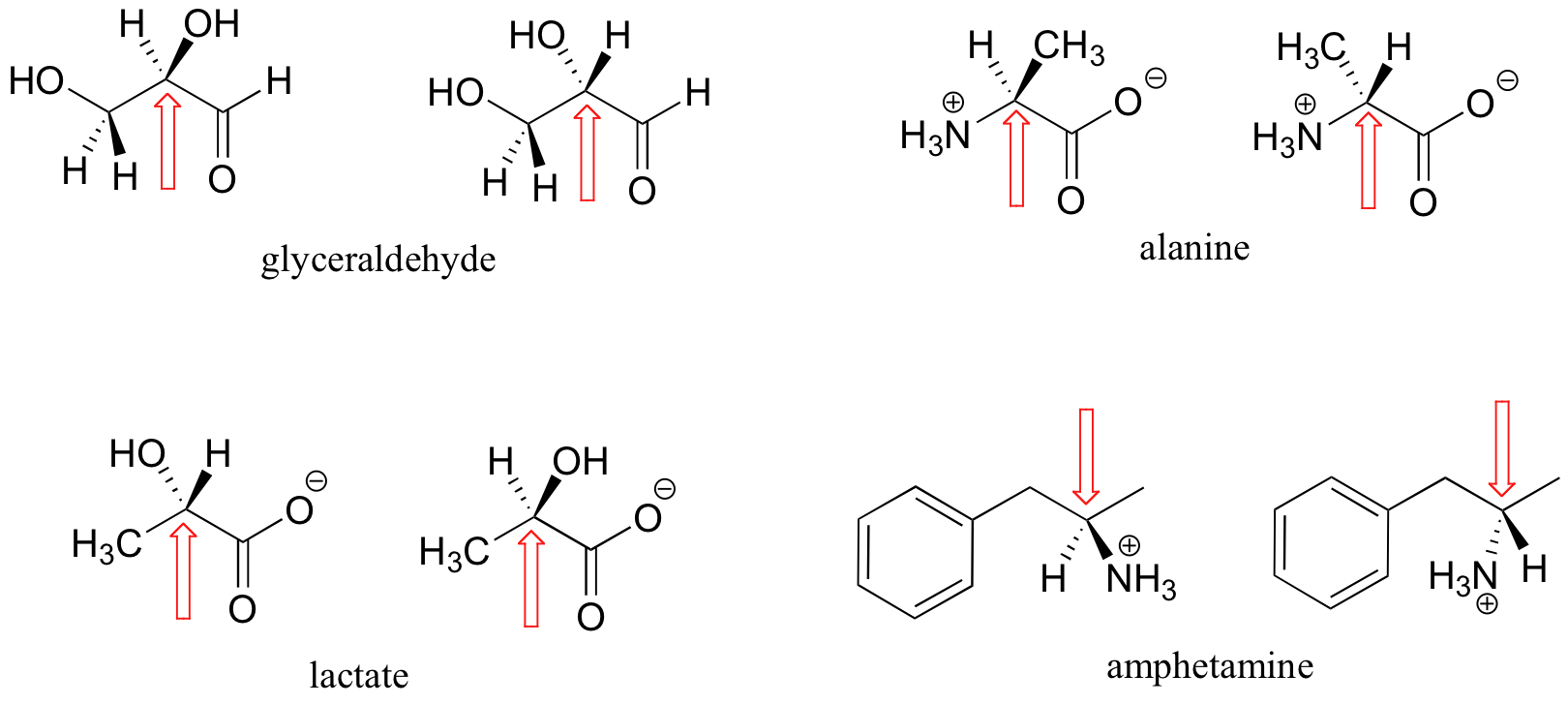
Here are some examples of molecules that are achiral (not chiral). Notice that none of these molecules has a stereocenter (an atom that is bound to four different substituents).
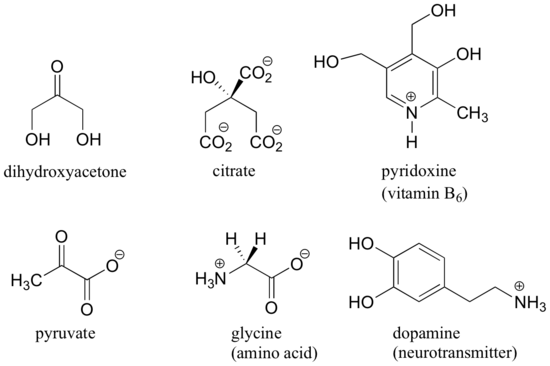
When evaluating a molecule for chirality, it is important to recognize that the use of the dashed/solid wedge drawing does not necessarily mean that the molecule is chiral. Chiral molecules are sometimes drawn without using wedges. Conversely, wedges may be used on carbons that are not stereocenters – look, for example, at the drawings of glycine and citrate in the figure above. Just because you see dashed and solid wedges in a structure, do not automatically assume that you are looking at a stereocenter.
Other elements in addition to carbon can be stereocenters. The phosphorus center of phosphate ion and organic phosphate esters, for example, is tetrahedral, and thus is potentially a stereocenter.

Having trouble visualizing chirality and enantiomers? It may be helpful to watch this
(Back to the Top)
5.9 The Importance of Chirality in Protein Interactions
The thalidomide that was used in the 1960s to treat depression and morning sickness was sold as a 50:50 mixture of both the R and the S enantiomer – this is referred to as a racemic mixture. A ‘squiggly’ bond in a chemical structure indicates a racemic mixture – thus racemic (R/S) thalidomide would be drawn as:
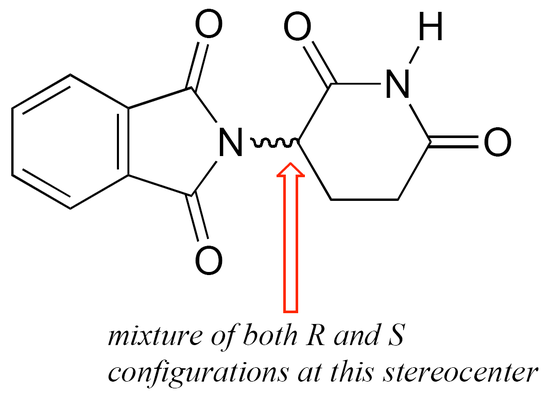
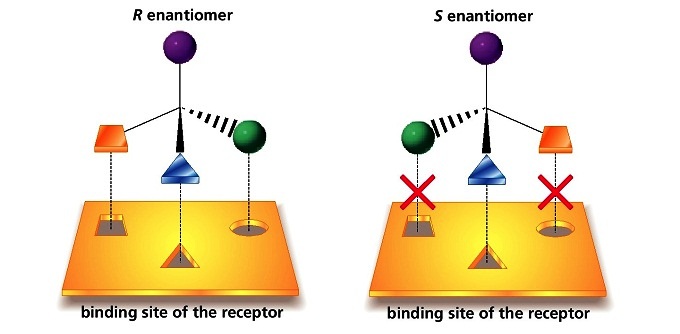
The problem with racemic thalidomide, as we learned above, is that only the R enantiomer is an effective medicine, while the S enantiomer causes mutations in the developing fetus. How does such a seemingly trivial structural variation lead to such a dramatic (and in this case, tragic) difference in biological activity? Virtually all drugs work by interacting in some way with important proteins in our cells: they may bind to pain receptor proteins to block the transmission of pain signals, for instance, or clog up the active site of an enzyme that is involved in the synthesis of cholesterol. Proteins are chiral molecules, and are very sensitive to stereochemistry: just as a right-handed glove won’t fit on your left hand, a protein that is able to bind tightly to (R)-thalidomide may not bind well at all to (S)-thalidomide (it will help to view a color version of the figure below).

Instead, it seems that (S)-thalidomide interacts somehow with a protein involved in the development of a growing fetus, eventually causing the observed birth defects.
Figure 5.22 Drug binding sites on proteins are stereospecific.
Source: www.kshitij-iitjee.com/Study/Chemistry/Part2/Chapter3/109.jpg
The over-the-counter painkiller ibuprofen is currently sold as a racemic mixture, but only the S enantiomer is effective.
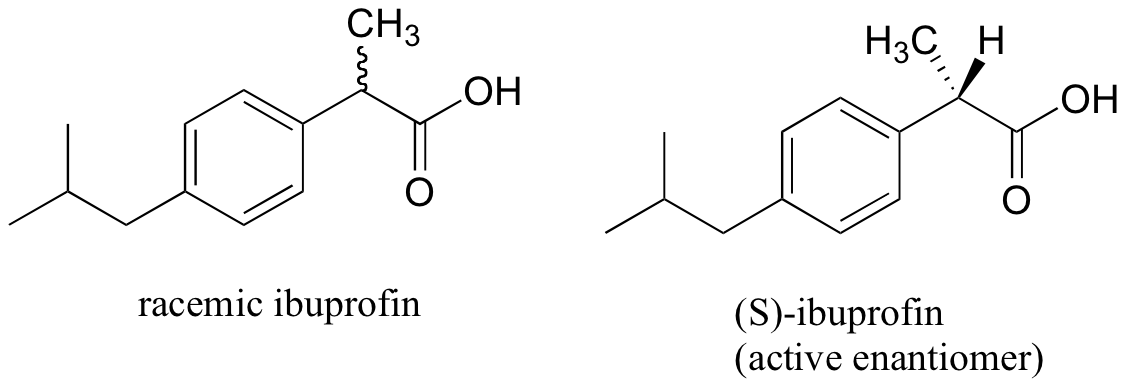
Fortunately, the R enantiomer does not produce any dangerous side effects, although its presence does seem to increase the amount of time that it takes for (S)-ibuprofen to take effect.
You can, with the assistance your instructor, directly experience the biological importance of stereoisomerism. Carvone is a chiral, plant-derived molecule that smells like spearmint in the R form and caraway (a spice) in the S form.
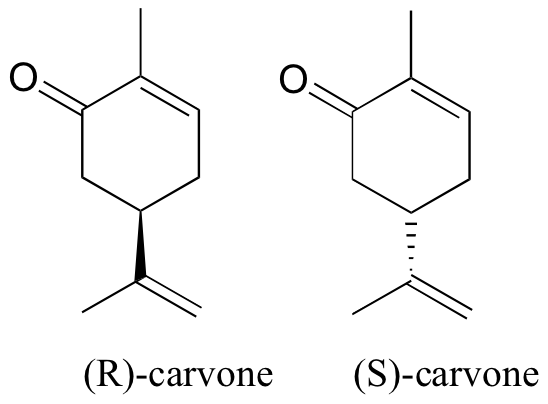
The two enantiomers interact differently with smell receptor proteins in your nose, generating the transmission of different chemical signals to the olfactory center of your brain.
(Back to the Top)
5.10 Common Organic Functional Groups
The number of known organic compounds is a quite large. In fact, there are many times more organic compounds known than all the other (inorganic) compounds discovered so far, about 7 million organic compounds in total. Fortunately, organic chemicals consist of a relatively few similar parts, combined in different ways, that allow us to predict how a compound we have never seen before may react, by comparing how other molecules containing the same types of parts are known to react.
These parts of organic molecules are called functional groups and are made up from specific bonding patterns with the atoms most commonly found in organic molecules (C, H, O, N, S, and P). The identification of functional groups and the ability to predict reactivity based on functional group properties is one of the cornerstones of organic chemistry.
Functional groups are specific atoms, ions, or groups of atoms having consistent properties. A functional group makes up part of a larger molecule.
For example, -OH, the hydroxyl group that characterizes alcohols, is an oxygen with a hydrogen attached. It could be found on any number of different molecules.
Just as elements have distinctive properties, functional groups have characteristic chemistries. An -OH functional group on one molecule will tend to react similarly, although perhaps not identically, to an -OH on another molecule.
Organic reactions usually take place at the functional group, so learning about the reactivities of functional groups will prepare you to understand many other things about organic chemistry.
Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. The structure of capsaicin, the fiery compound found in hot peppers, incorporates several functional groups, labeled in the figure below and explained throughout this section.
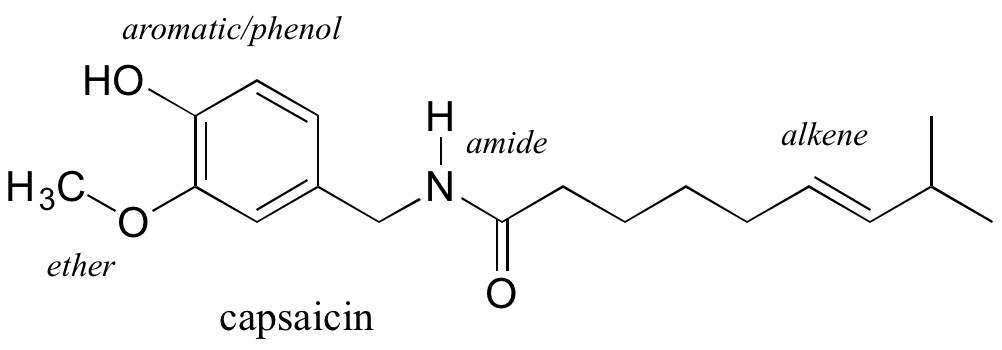
As we progress in our study of organic chemistry, it will become extremely important to be able to quickly recognize the most common functional groups, because they are the key structural elements that define how organic molecules react. For now, we will only worry about drawing and recognizing each functional group, as depicted by Lewis and line structures. Much of the remainder of your study of organic chemistry will be taken up with learning about how the different functional groups behave in organic reactions. Below is a brief introduction to the major organic functional groups.
(Back to the Top)
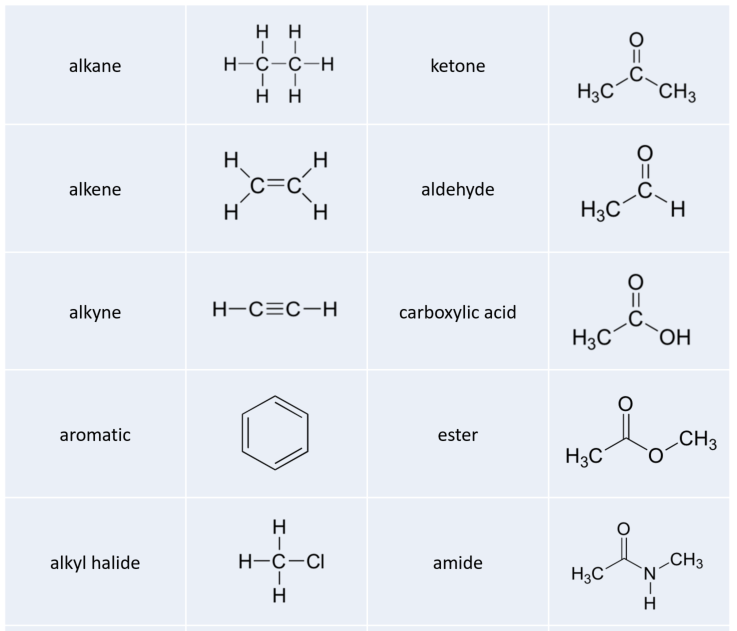
Alkanes
The ‘default’ in organic chemistry (essentially, the lack of any functional groups) is given the term alkane, characterized by single bonds between carbon and carbon, or between carbon and hydrogen. Methane, CH4, is the natural gas you may burn in your furnace. Octane, C8H18, is a component of gasoline.
Alkenes and Alkynes
Alkenes (sometimes called olefins) have carbon-carbon double bonds, and alkynes have carbon-carbon triple bonds. Ethene, the simplest alkene example, is a gas that serves as a cellular signal in fruits to stimulate ripening. (If you want bananas to ripen quickly, put them in a paper bag along with an apple – the apple emits ethene gas (also called ethylene), setting off the ripening process in the bananas). Ethyne, commonly called acetylene, is used as a fuel in welding blow torches.
Many alkenes can take two geometric forms: cis or trans. The cis and trans forms of a given alkene are different isomers with different physical properties because there is a very high energy barrier to rotation about a double bond. In the example below, the difference between cis and trans alkenes is readily apparent.
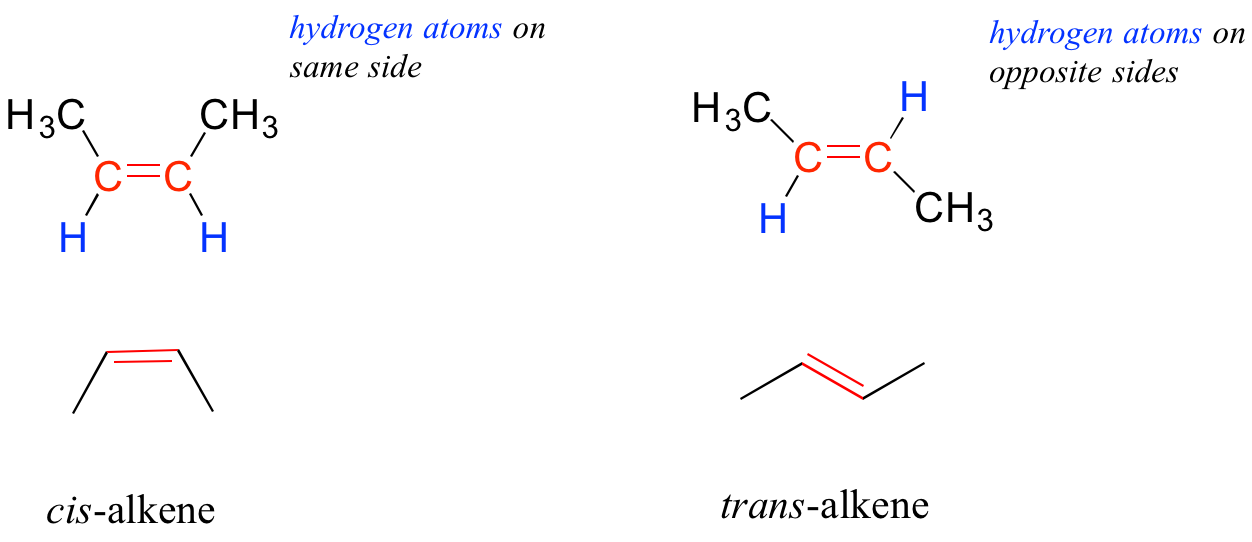
Alkanes, alkenes, and alkynes are all classified as hydrocarbons, because they are composed solely of carbon and hydrogen atoms. Alkanes are said to be saturated hydrocarbons, because the carbons are bonded to the maximum possible number of hydrogens – in other words, they are ‘saturated’ with hydrogen atoms. The double and triple-bonded carbons in alkenes and alkynes have fewer hydrogen atoms bonded to them – they are thus referred to as unsaturated hydrocarbons.
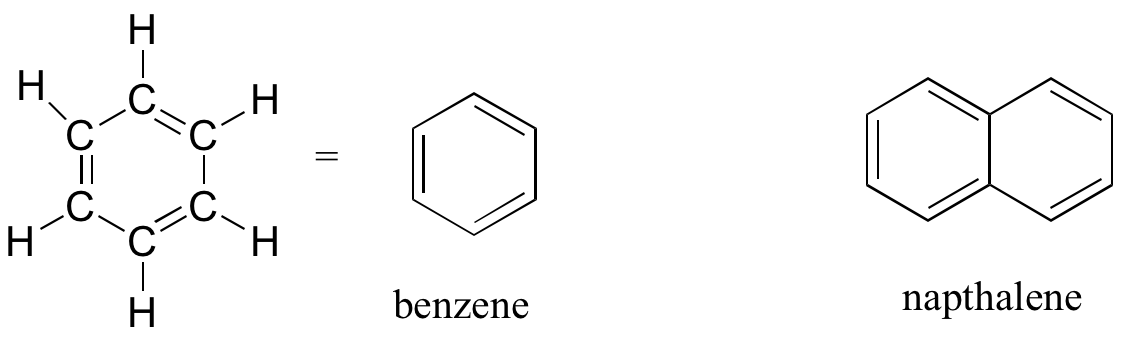
Aromatics
The aromatic group is exemplified by benzene (which used to be a commonly used solvent on the organic lab, but which was shown to be carcinogenic), and naphthalene, a compound with a distinctive ‘mothball’ smell. Aromatic groups are planar (flat) ring structures, and are widespread in nature.
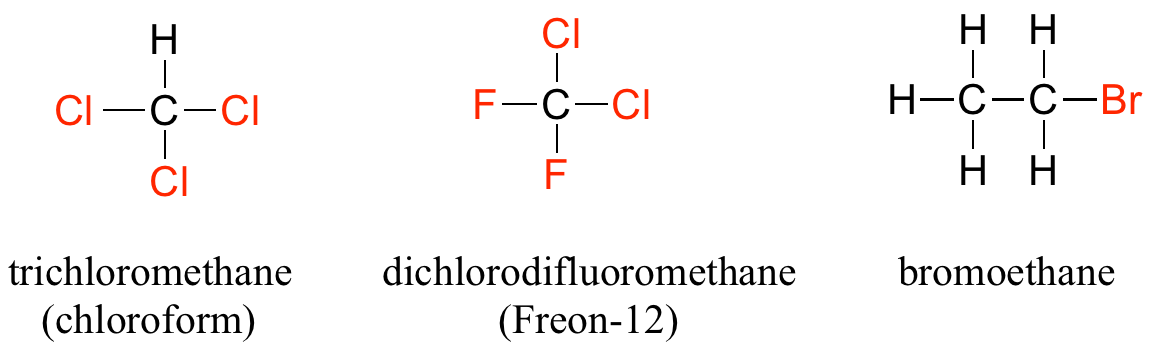
When the carbon of an alkane is bonded to one or more halogens, the group is referred to as an alkyl halide or haloalkane. Chloroform is a useful solvent in the laboratory, and was one of the earlier anesthetic drugs used in surgery. Chlorodifluoromethane was used as a refrigerant and in aerosol sprays until the late twentieth century, but its use was discontinued after it was found to have harmful effects on the ozone layer. Bromoethane is a simple alkyl halide often used in organic synthesis. Alkyl halides groups are quite rare in biomolecules.
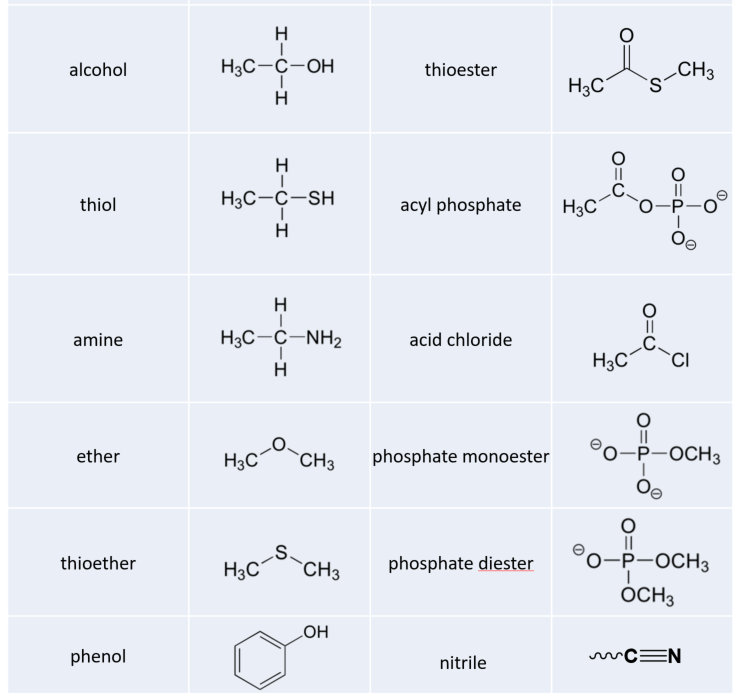
Alcohols, Phenols, and Thiols
In the alcohol functional group, a carbon is single-bonded to an OH group (the OH group, when it is part of a larger molecule, is referred to as a hydroxyl group). Except for methanol, all alcohols can be classified as primary, secondary, or tertiary. In a primary alcohol, the carbon bonded to the OH group is also bonded to only one other carbon. In a secondary alcohol and tertiary alcohol, the carbon is bonded to two or three other carbons, respectively. When the hydroxyl group is directly attached to an aromatic ring, the resulting group is called a phenol. The sulfur analog of an alcohol is called a thiol (from the Greek thio, for sulfur).

Note that the definition of a phenol states that the hydroxyl oxygen must be directly attached to one of the carbons of the aromatic ring. The compound below, therefore, is not a phenol – it is a primary alcohol.
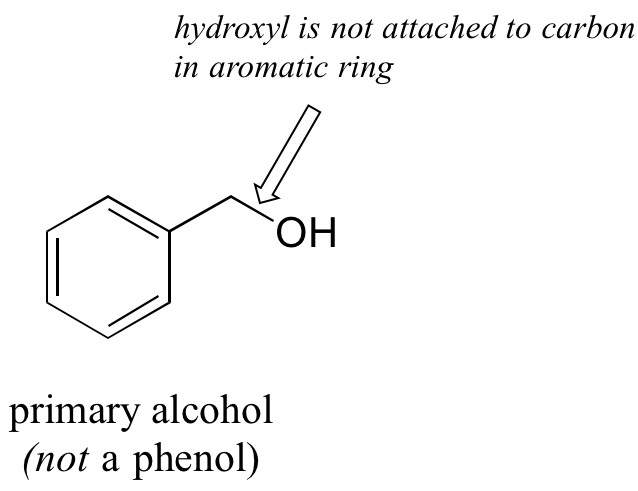
The distinction is important, because there is a significant difference in the reactivity of alcohols and phenols
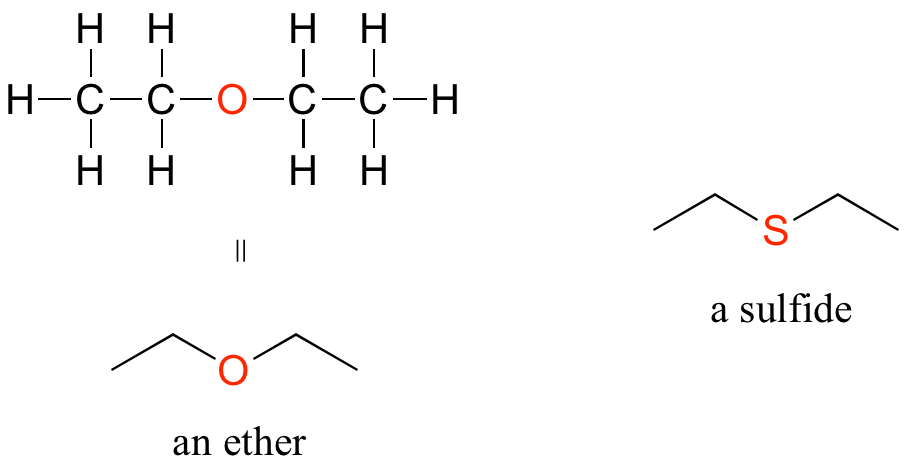
Ethers and Sulfides
In an ether functional group, an oxygen is bonded to two carbons. Below is the structure of diethyl ether, a common laboratory solvent and also one of the first compounds to be used as an anesthetic during operations. The sulfur analog of an ether is called a thioether or sulfide.
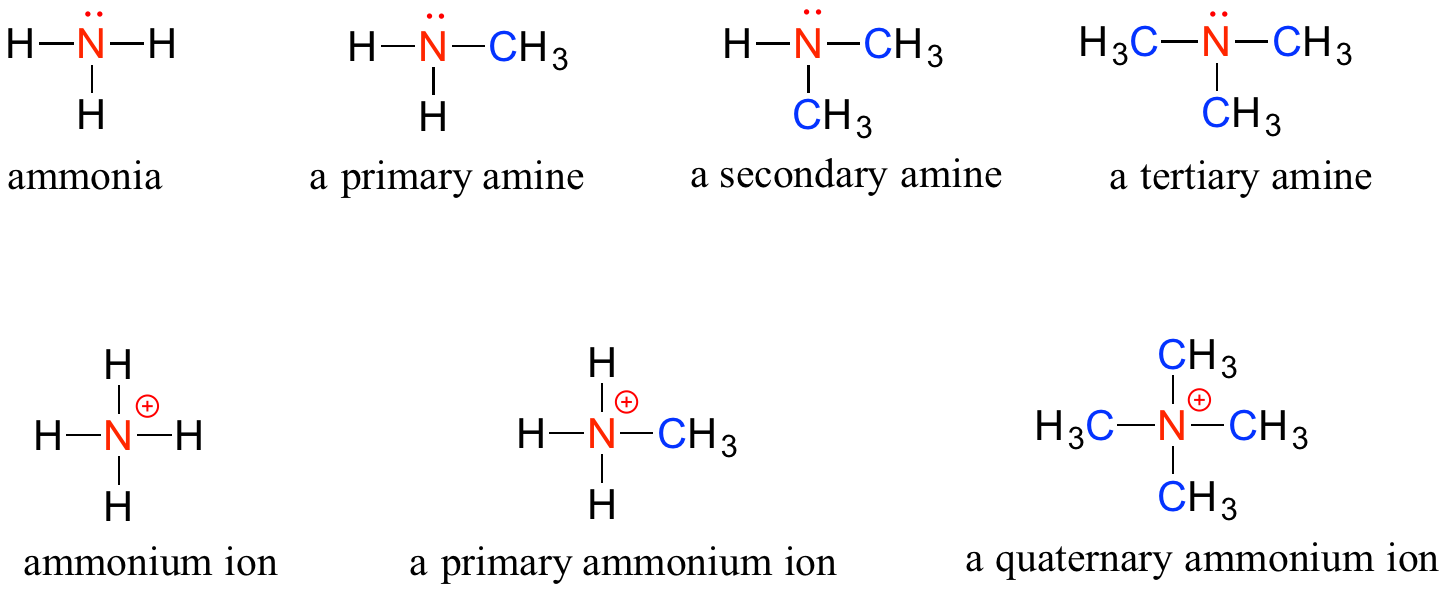
Amines
Amines are characterized by nitrogen atoms with single bonds to hydrogen and carbon. Just as there are primary, secondary, and tertiary alcohols, there are primary, secondary, and tertiary amines. Ammonia is a special case with no carbon atoms.
One of the most important properties of amines is that they are basic, and are readily protonated to form ammonium cations. In the case where a nitrogen has four bonds to carbon (which is somewhat unusual in biomolecules), it is called a quaternary ammonium ion.
Note: Do not be confused by how the terms ‘primary’, ‘secondary’, and ‘tertiary’ are applied to alcohols and amines – the definitions are different. In alcohols, what matters is how many other carbons the alcohol carbon is bonded to, while in amines, what matters is how many carbons the nitrogen is bonded to.
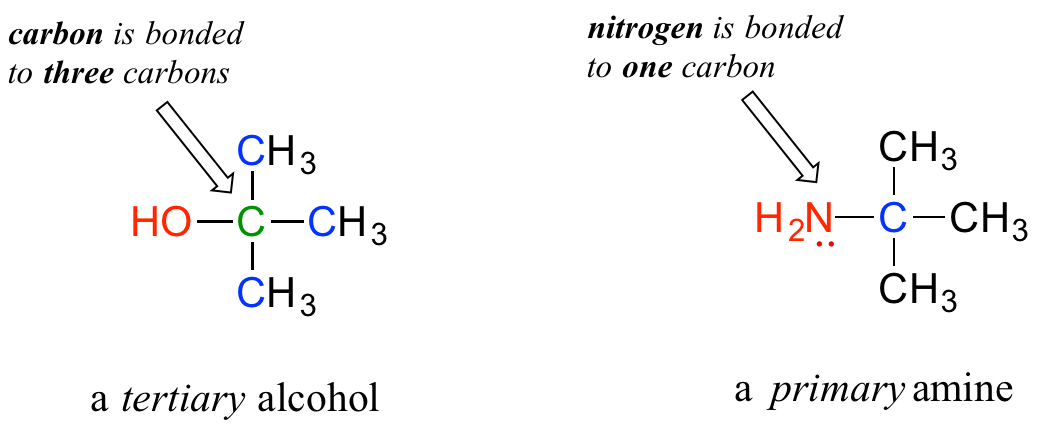
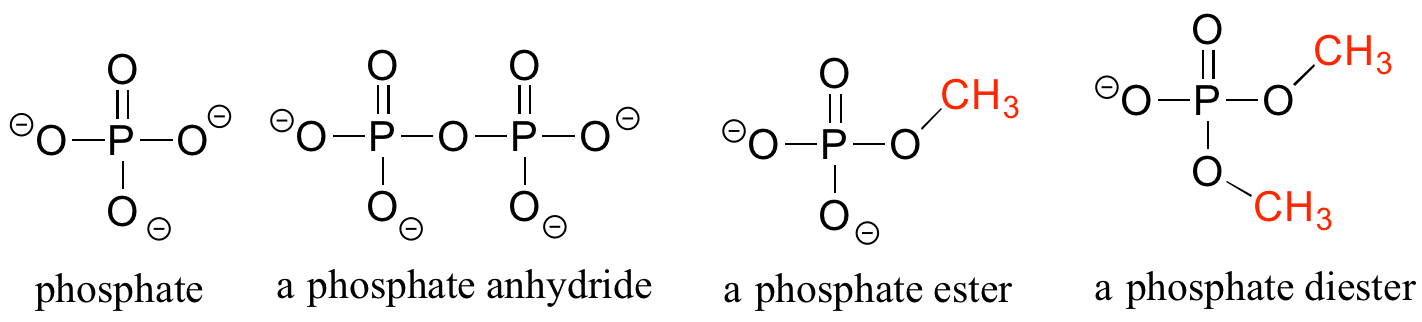
Organic Phosphates
Phosphate and its derivative functional groups are ubiquitous in biomolecules. Phosphate linked to a single organic group is called a phosphate ester; when it has two links to organic groups it is called a phosphate diester. A linkage between two phosphates creates a phosphate anhydride.
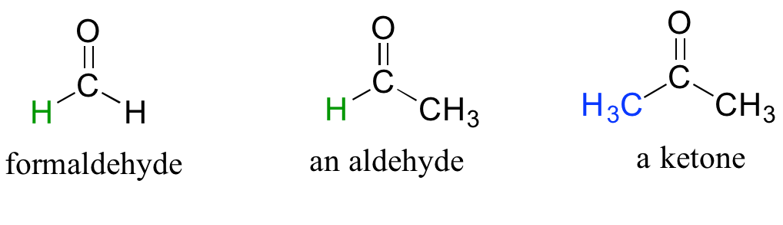
Aldehydes and Ketones
There are a number of functional groups that contain a carbon-oxygen double bond, which is commonly referred to as a carbonyl. Ketones and aldehydes are two closely related carbonyl-based functional groups that react in very similar ways. In a ketone, the carbon atom of a carbonyl is bonded to two other carbons. In an aldehyde, the carbonyl carbon is bonded on one side to a hydrogen, and on the other side to a carbon. The exception to this definition is formaldehyde, in which the carbonyl carbon has bonds to two hydrogens.
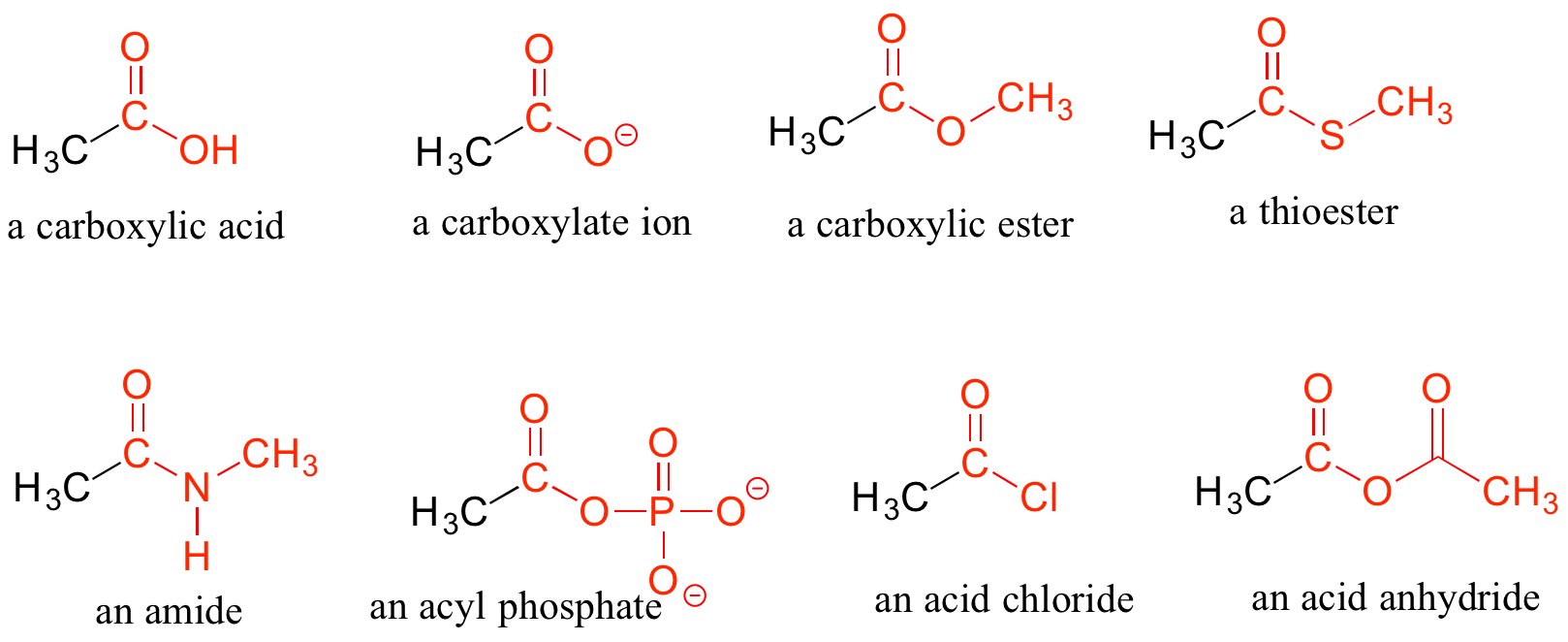
Carboxylic Acids and Their Derivatives
When a carbonyl carbon is bonded on one side to a carbon (or hydrogen) and on the other side to an oxygen, nitrogen, or sulfur, the functional group is considered to be one of the ‘carboxylic acid derivatives’, a designation that describes a set of related functional groups. The main member of this family is the carboxylic acid functional group, in which the carbonyl is bonded to a hydroxyl group. The carboxylate ion form has donated the H+ to the solution. Other derivatives are carboxylic esters (usually just called ‘esters’), thioesters, amides, acyl phosphates, acid chlorides, and acid anhydrides. With the exception of acid chlorides and acid anhydrides, the carboxylic acid derivatives are very common in biological molecules and/or metabolic pathways and will be discussed in further details in a later chapter.
Practice Recognizing Functional Groups in Molecules
A single compound often contains several functional groups, particularly in biological organic chemistry. The six-carbon sugar molecules glucose and fructose, for example, contain aldehyde and ketone groups, respectively, and both contain five alcohol groups. A compound with several alcohol groups is often referred to as a ‘polyol’.
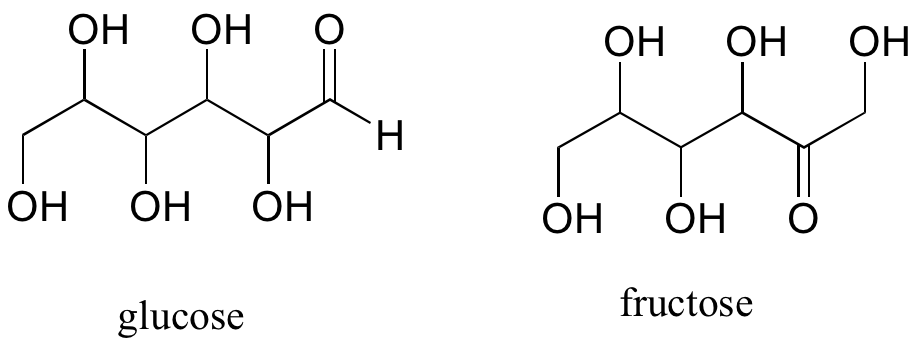
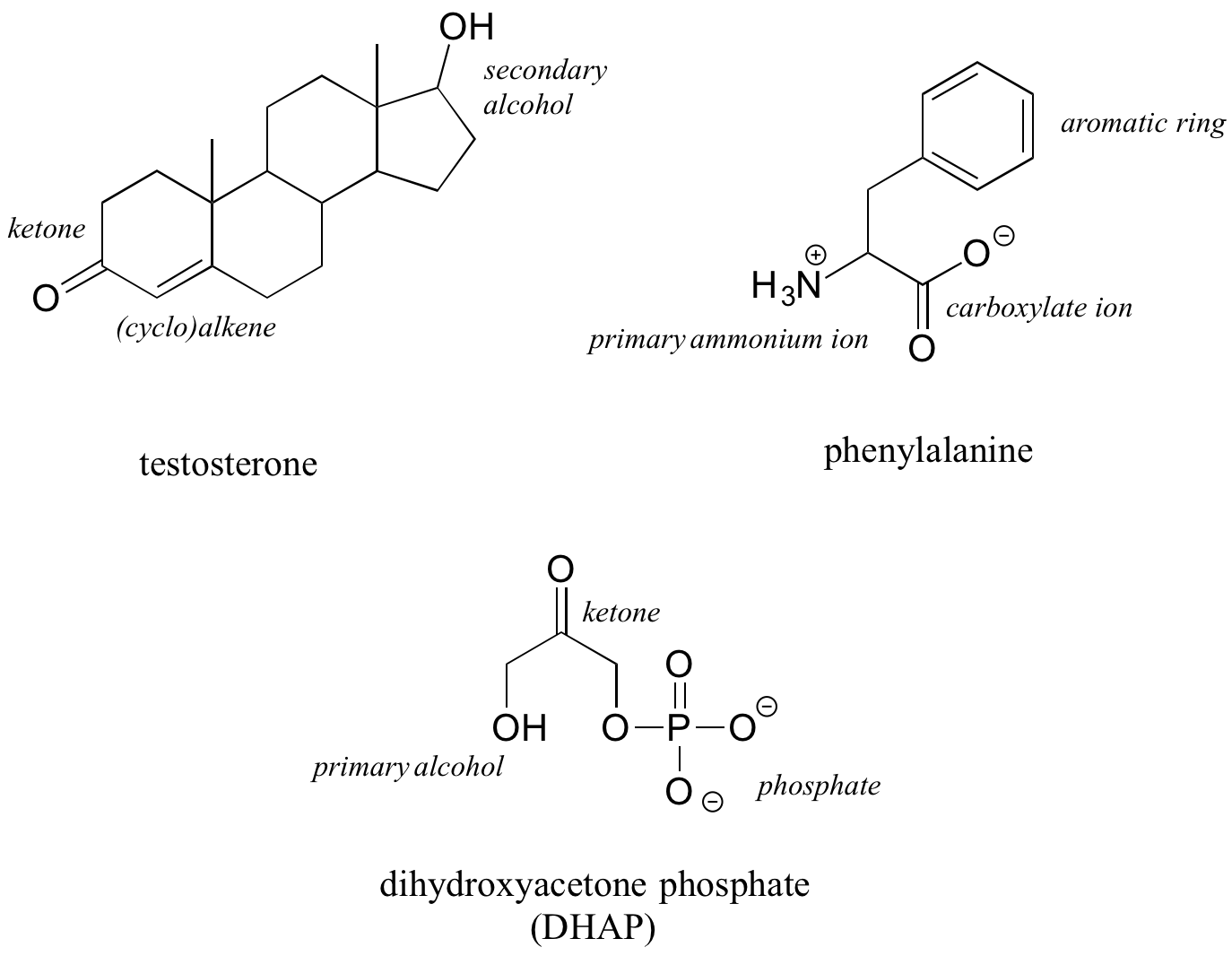
The hormone testosterone, the amino acid phenylalanine, and the glycolysis metabolite dihydroxyacetone phosphate all contain multiple functional groups, as labeled below.
While not in any way a complete list, this section has covered most of the important functional groups that we will encounter in biochemistry. Table 5.7 provides a summary of all of the groups listed in this section.
Table 5.7 Common Organic Functional Groups
Exercise 5.10.1
Identify the functional groups (other than alkanes) in the following organic compounds. State whether alcohols and amines are primary, secondary, or tertiary.
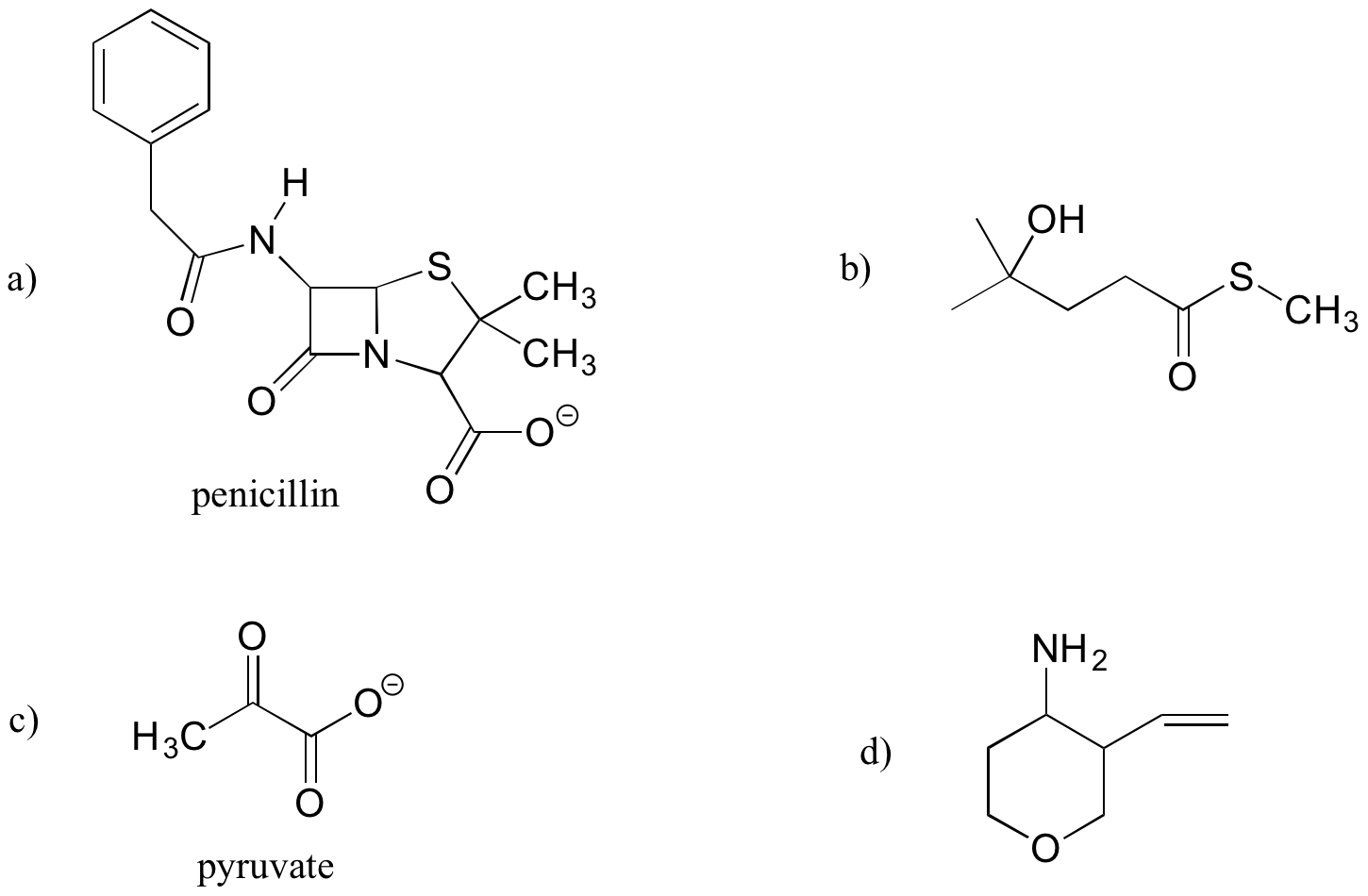
Exercise 5.10.2
Draw one example each of compounds fitting the descriptions below, using line structures. Be sure to designate the location of all non-zero formal charges. All atoms should have complete octets (phosphorus may exceed the octet rule). There are many possible correct answers for these, so be sure to check your structures with your instructor or tutor.
a) a compound with molecular formula C6H11NO that includes alkene, secondary amine, and primary alcohol functional groups
b) an ion with molecular formula C3H5O6P2- that includes aldehyde, secondary alcohol, and phosphate functional groups.
c) A compound with molecular formula C6H9NO that has an amide functional group, and does not have an alkene group.
(back to the top)
5.11 Chapter Summary
Atoms can share pairs of valence electrons to obtain a valence shell octet. This sharing of electrons is a covalent bond. A species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each type in the molecule. The two electrons shared in a covalent bond are called a bonding pair of electrons. The electrons that do not participate in covalent bonds are called nonbonding pairs (or lone pairs) of electrons. A covalent bond consisting of one pair of shared electrons is called a single bond.
Covalent bonds occur between nonmetal atoms. Naming simple covalent compounds follows simple rules similar to those for ionic compounds. However, for covalent compounds, numerical prefixes are used as necessary to specify the number of atoms of each element in the compound.
In some cases, more than one pair of electrons is shared to satisfy the octet rule. Two pairs of electrons are shared by two atoms to make a double bond. Three pairs of atoms are shared to make a triple bond. Single, double, and triple covalent bonds may be represented by one, two, or three dashes, respectively, between the symbols of the atoms. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons.
To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. The greater the electronegativity difference between the atoms involved in the covalent bond, the more polarity the bond displays.
In comparison to ionic compounds, covalent molecules tend to have lower melting and boiling points, are less soluble in water, and are poor conductors of electricity. These major differences are largely due to increased polarity of ionic bonds when compared with covalent bonds.
Organic molecules can be represented in a number of different ways. You should be able to recognize and draw out organic structures in each of these different structural representations: molecular formula, a displayed formula, a partially-condensed and fully-condensed structure, and line structures (with wedges and dashes when appropriate). In addition, regions of an organic structure may represented by an R-group, to save time in structure recreation. This is especially useful when drawing a group of related compounds that only differ in one or two regions. The differing regions of the molecule can be written out as R-groups to avoid having to redraw the entire molecule each time.
Organic molecules can have isomer structures. Structural or constitutional isomers share the same molecular formula but the atoms within the structure are bonded together in a different orientation. Stereoisomers have the same molecular formula and the atoms are also bonded together in the same order, however, the 3-dimentional arrangement of the atoms in space is different. A special type of stereoisomer is called an enantiomer. Enantiomers are stereoisomers that are mirror images of eachother, but are not superimposable. Enantiomers have most of the same physical and chemical properties, however, since biological interactions depend on the 3-dimensional structure of molecules, enantiomers often have different biological activities. Molecules that are mirror images, but are not superimposable are said to have the property of chirality or handedness. Carbon displays chirality when it has four different substituents on it.
Functional groups are structural units within organic compounds that are defined by specific bonding arrangements between specific atoms. Just as elements have distinctive properties, functional groups have characteristic chemistries. An -OH functional group on one molecule will tend to react similarly, although not identically, to an -OH group on another molecule. Functional groups are the key structural elements that define how organic molecules react, thus it is important to learn how to recognize common organic functional groups.
(back to the top)
5.12 References
Chapter 5 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
4. Molecules and Molecular Compounds. (2017) Libretexts. Available at: https://chem.libretexts.org/Textbook_Maps/General_Chemistry_Textbook_Maps/Map%3A_Chemistry%3A_The_Central_Science_(Brown_et_al.)/02._Atoms,_Molecules,_and_Ions/2.6%3A_Molecules_and_Molecular_Compounds
5. Clark, J. (2017) ‘General Principles of Chemical Bonding’ Published by Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Coordinate_(Dative_Covalent)_Bonding
6. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12.
7. Wikipedia, Ionic Compound. Available at: https://en.wikipedia.org/wiki/Ionic_compound
8. Physical and Theoretical Chemistry (2017) Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds_vs_Ionic_Bonds.
9. Wikibooks. (2015) Organic Chemistry. Available at: https://en.wikibooks.org/wiki/Organic_Chemistry.
10. Clark, J. (2014) How to Draw Organic Molecules. Available at: http://chem.libretexts.org/Core/Organic_Chemistry/Fundamentals/How_to_Draw_Organic_Molecules
11. Soderberg, T. (2016). Organic Chemistry With a Biological Emphasis . Published under Creative Commons by-nc-sa 3.0.
12. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
13. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
14. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
15. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
16. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12.
17. Wikipedia, Ionic Compound. Available at: https://en.wikipedia.org/wiki/Ionic_compound