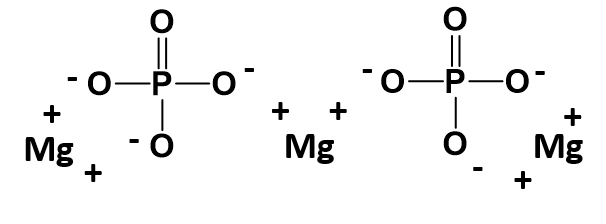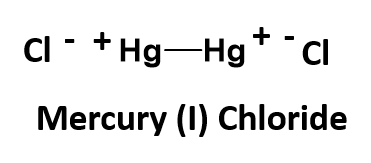Home » Student Resources » Online Chemistry Textbooks » CH103: Allied Health Chemistry » CH103 – CHAPTER 4: Ions and Ionic Compounds
MenuCH103: Allied Health Chemistry
Chapter 4 – Ions and Ionic Compounds
4.1 Introduction to the Octet Rule
4.2 Ions and the Periodic Table
Common Cations
Common Anions
Ions of Transition Metals
4.3 Ionic Bonding
4.4 Practice Writing Correct Ionic Formulas
4.5 Naming Ions and Ionic Compounds
4.6 Polyatomic Ions
4.7 Naming Polyatomic Ions
4.8 Properties and Types of Ionic Compounds
4.9 Arrhenius Acids and Bases
4.10 Ions, Neurons, and Action Potentials
4.11 Chapter Summary
4.12 References
4.1 Introduction to the Octet Rule
Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). The overall charge on the atom is zero, because the magnitude of the negative charge is the same as the magnitude of the positive charge. This one-to-one ratio of charges is not, however, the most common state for many elements. Deviations from this ratio result in charged particles called ions.
Throughout nature, things that are high in energy tend to move toward lower energy states. Lower energy configurations are more stable, so things are naturally drawn toward them. For atoms, these lower energy states are represented by the noble gas elements. These elements have electron configurations characterized by full valence electron configurations. This makes them stable and unreactive. They are already at a low energy state, so they tend to stay as they are.
The elements in the other groups have valence electron configurations that are not full, so they are unstable when compared to the noble gases. This instability drives them toward the lower energy states represented by the noble gases that are nearby in the periodic table. In these lower energy states, the outermost energy level has eight electrons (an “octet”). The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the “Octet Rule.”
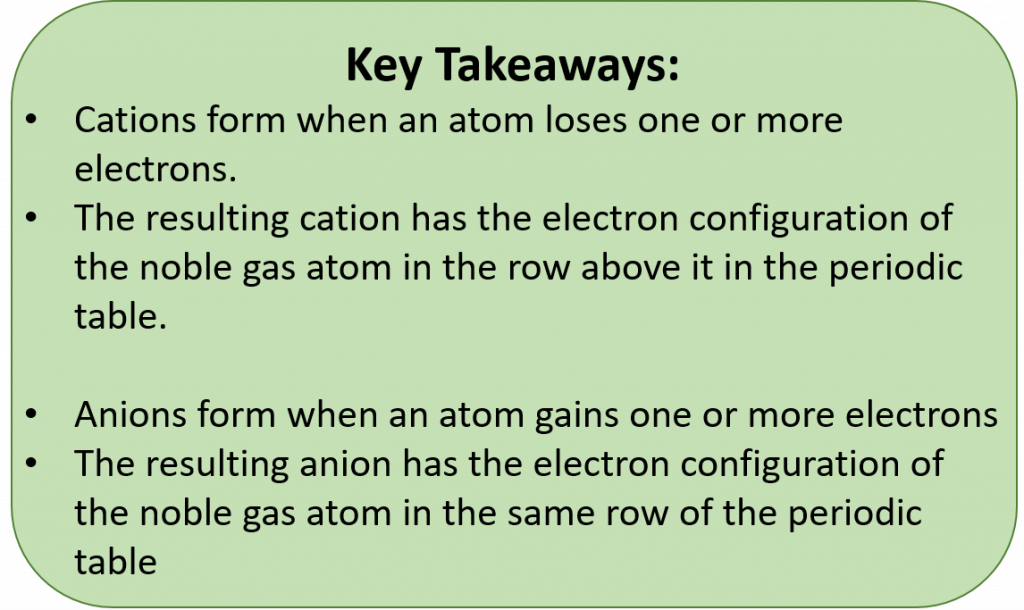
There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. One way is the transfer of electrons between two atoms until both atoms have octets. Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but with the transfer of electrons the individual atoms acquire a nonzero electric charge. Those that lose electrons become positively charged, and those that gain electrons become negatively charged. Recall that atoms carrying positive or negative charges are called ions. If an atom has gained one or more electrons, it is negatively charged and is called an anion. If an atom has lost one or more electrons, it is positively charged and is called a cation. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds.
The second way for an atom to obtain an octet of electrons is by sharing electrons with another atom. These shared electrons simultaneously occupy the outermost shell of both atoms. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Chapter 4 “Covalent Bonding and Simple Molecular Compounds”.
Electron-Dot Symbols
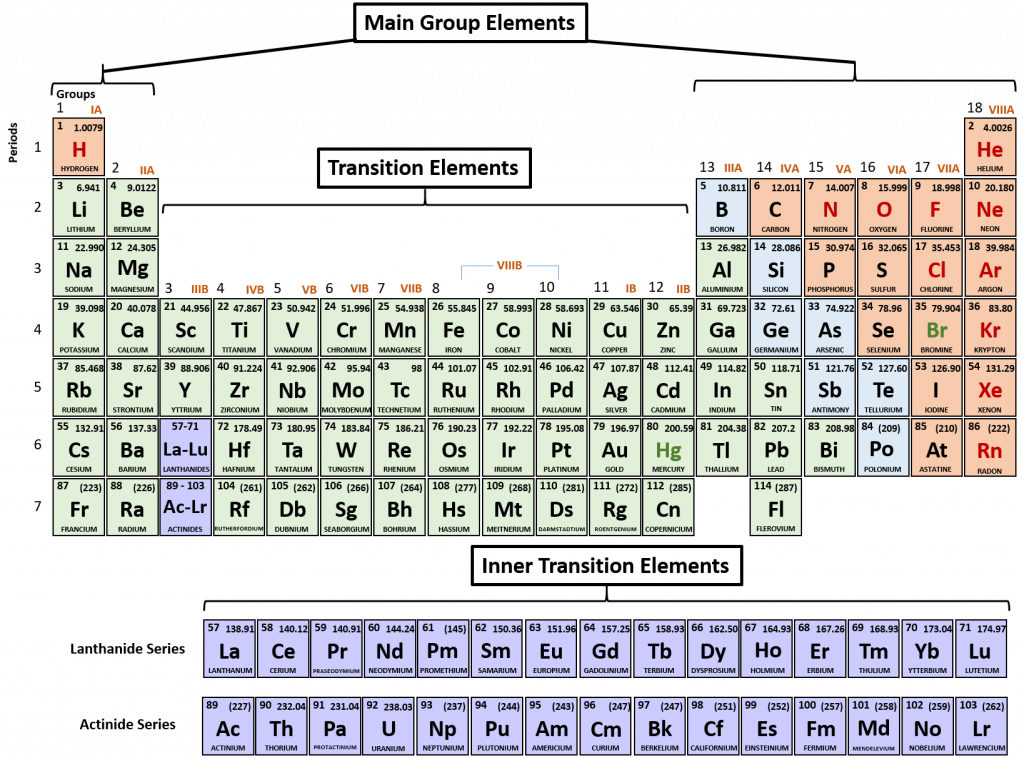
For each element on the periodic table, it is possible to predict the number of valence shell electrons that they will contain. When looking at the periodic table, it it divided into main group elements and transition elements. The main group elements are numbered IA to VIIIA, and their number of valance shell electrons correspond to their group number (Fig 4.1). For example all of the elements in the halogen family belong to group VIIA and correspondingly have 7 electrons in their valence shell. For all of the transition elements and the inner transition elements, they have a total of 2 electrons in their valence shell.
Figure 4.1 Periodic Table of the Elements. Main group, transition and inner transition elements are indicated.
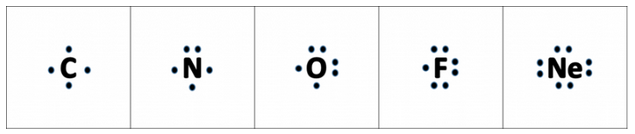
Although it is entirely possible to define the number of valence electrons in an atom through numbers, sometimes it is helpful to have a graphical representation. The graphical notation used for valence electrons is called an Electron-Dot Symbol. To draw an electron dot symbol, start with the abbreviation for the element of interest as the center, signifying the nucleus of the atom. From there, identify the number of valence electrons the atom has according to its position on the periodic table, and then add a single dot for each valence electron around the element (Fig. 4.2). Students often want to place these electron dots around the element randomly, but it is useful to use the four cardinal directions as a guide. First place single electrons around the atom in each of the four cardinal positions until you run out of electrons. For elements that have more than four valence electrons, you will begin pairing them in the four cardinal directions. Note that the noble gases have complete octets and will have a total of 8 electrons in their valence shell (Fig 4.2).
Figure 4.2 Electron Dot Symbols. Electron Dot Symbols are shown for Carbon through Neon on the Periodic Table. Note that a single electron will be placed in each of the four cardinal directions before electrons will be paired with another electron.
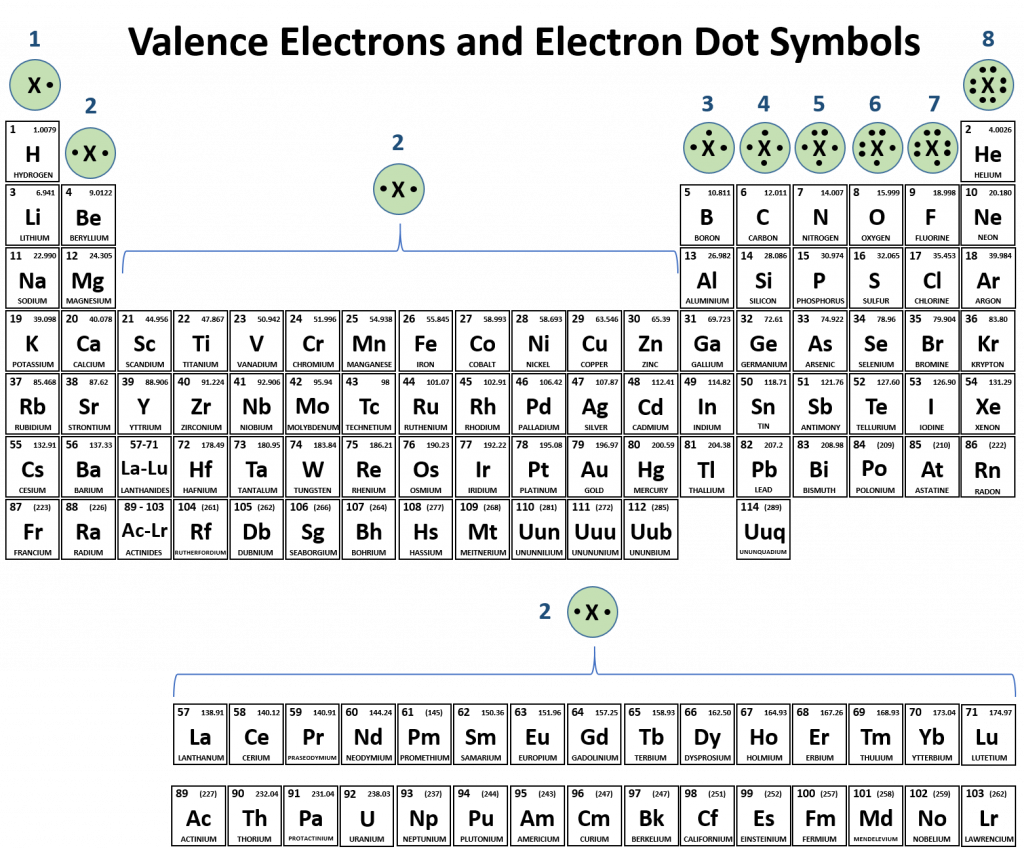
Overall, the periodic table can be used as a guide for determining the number of valence electrons for each element (Fig 4.3).
Figure 4.3 Periodic Table with Electron Dot Symbols. Electron dot symbols are drawn above each family or group of elements on the periodic table, where X indicates any element within that family or group.
(Back to the Top)
4.2 Ions and the Periodic Table
The elements on the right side of the periodic table, nonmetals, gain the electrons necessary to reach the stable electron configuration of the nearest noble gas. Elements on the left side of the periodic table, metals, lose the electrons necessary to reach the electron configuration of the nearest noble gas. Transition elements can vary in how they move toward lower energy configurations.
Common Cations
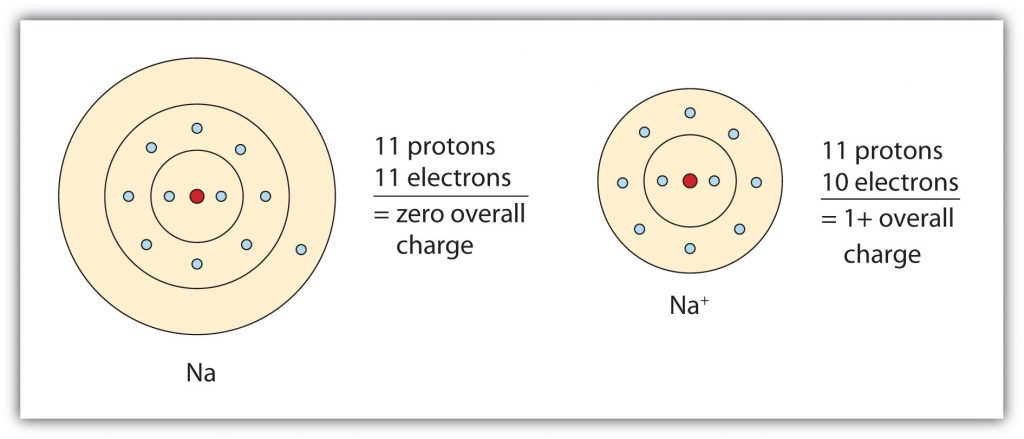
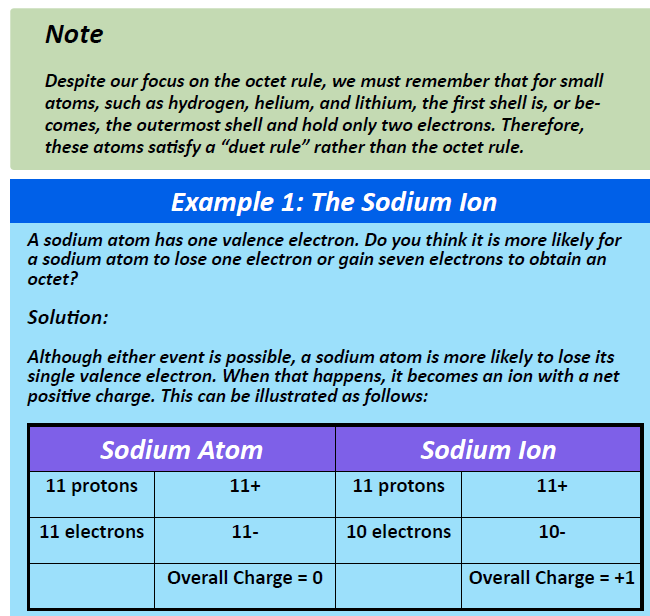
Group IA elements form ions with a +1 charge. They lose one electron upon ionization, moving into the electron configuration of the previous noble gas. For example as shown in Figure 4.4, when a sodium (Na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (Na+) with the electron configuration that looks like the previous noble gas, neon. The sodium ion has one fewer electron than it has protons, so it has a single positive charge and is called a cation.
Figure 4.4 The Formation of a Sodium Ion. Sodium tends to lose it’s valence shell electron in the third shell during ionic bond formation. It is left with a full octet in the second shell and now has the electron configuration of neon. Note that it still has the same number of protons (11) as the original sodium atom and retains the identity of sodium. However, there are now only 10 electrons within the electron cloud, resulting in a net positive (+1) charge.
Upon losing that electron, the sodium ion now has an octet of electrons from the second principal energy level. The electron configuration of the sodium ion is now the same as that of the noble gas neon. The term isoelectronic refers to an atom and an ion of a different atom (or two different ions) that have the same electron configuration. The sodium ion is isoelectronic with the neon atom.
Overall, Group IA elements will lose one electron to reach the electron state of the noble gas preceding them in the periodic table. Note that the nucleus of the atom remains unchanged and thus, the identity of the ion is also unchanged. A sodium ion has the same electron configuration as neon, but not the same proton/neutron configuration. Thus, it retains its identity as the element, sodium even when it has undergone the loss of an electron. Similarly, Group IIA elements lose two valence electrons to form ions with a +2 charge and Group IIIA elements lose three electrons to form ions with a +3 charge. This gives them the electron configuration of the noble gas that comes before them in the periodic table.
While hydrogen is in the first column, it is not considered to be an alkali metal, and so it does not fall under the same classification as the elements below it in the periodic table. This is because hydrogen is very small and can only house a total of 2 electrons to become filled. It is an exception to the Octet Rule. Thus, instead of following the octet rule, it reaches greater stability by gaining a “duet” of electrons through bonding with other atoms. Thus, hydrogen can form both covalent bonds and ionic bonds, depending on the element that it is interacting with. When it participates in ionic bonds, it most often will lose its electron forming a +1 cation. Note, that hydrogen only has one electron to begin with, so when it loses an electron in the ionized state, there is only a single proton left in the nucleus of the atom. Thus, when hydrogen is ionized to H+ it is often referred to as a proton. It can also be ionized, forming a -1 anion. In this case, the H– anion is named using standard convention forming the hydride ion. During the ionization of hydrogen, the H+ state is more common than the H– state. In addition, the H+ ion is very important in the chemistry of acids. Acids are defined as compounds that donate H+ ions in aqueous solutions.
Cations are named very simply by following the element name with the word ‘ion’. Thus, a sodium atom that has lost electrons, is now referred to as a sodium ion.
Common Anions
Elements on the other side of the periodic table, the nonmetals, tend to gain electrons in order to reach the stable electron configurations of the noble gases that come after them in the periodic table.
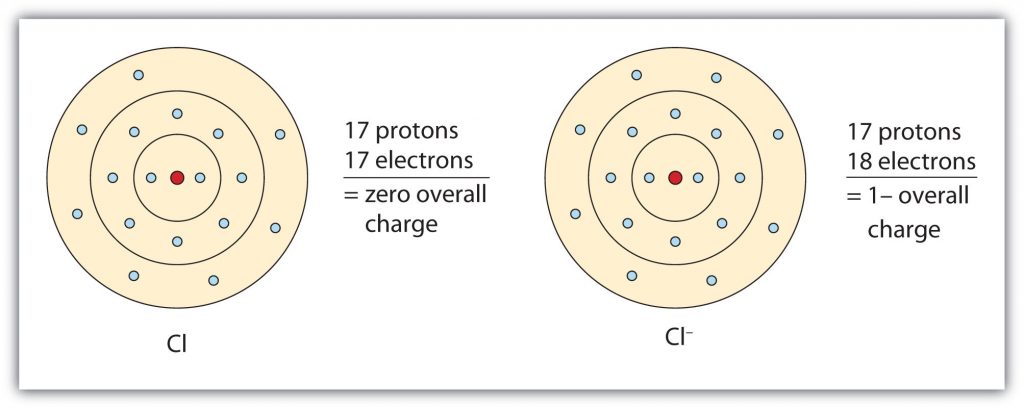
Group VIIA elements gain one electron when ionized, obtaining a -1 charge. For example as shown in Figure 4.5, chlorine (Cl), when ionized, gains an electron to reach the electron configuration of the noble gas that follows it in the periodic table, argon. This gives it a single negative charge, and it is now a chloride ion (Cl–); note the slight change in the suffix (-ide instead of -ine) to create the name of this anion.
Fig 4.5 The Formation of a Chloride Ion. On the left, a chlorine atom has 17 electrons. On the right, the chloride ion has gained an extra electron for a total of 18 electrons and a 1– charge. Note that the chloride ion has now filled its outer shell and contains eight electrons, satisfying the octet rule.
Group VIA elements gain two electrons upon ionization, obtaining -2 charges and reaching the electron configurations of the noble gases that follow them in the periodic table. Whereas, Group VA elements gain three electrons, obtaining -3 charges and also reaching the electron configurations of the noble gases that follow in the periodic table.
It is important not to misinterpret the concept of being isoelectronic. A sodium ion is very different from a neon atom because the nuclei of the two contain different numbers of protons. One is an essential ion that is a part of table salt, while the other is an unreactive gas that is a very small part of the atmosphere. Likewise, sodium ions are very different than magnesium ions, fluoride ions, and all the other members of the neon isoelectronic series (N3−,O2−,F−,Ne,Na+,Mg2+,Al3+)
.
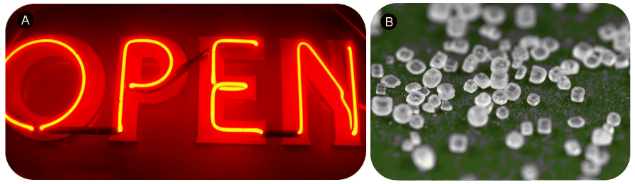
Figure 4.6: Isoelectric Atoms Have Different Properties. Neon gas (A) and sodium chloride crystals (B). Neon atoms and sodium ions are isoelectronic. Neon is a colorless and unreactive gas that glows a distinctive red-orange color in a gas discharge tube. Sodium ions are commonly found in crystals of salt such as sodium chloride, ordinary table salt.
Ions of Transition and Inner Transition Metals
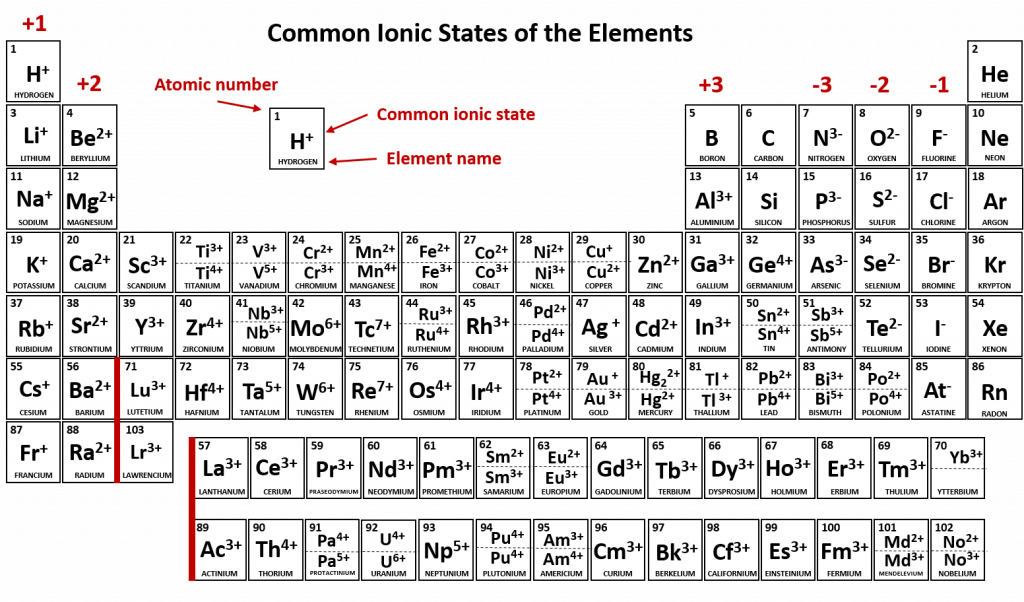
The transition and inner transition metals are an interesting and challenging group of elements. They have perplexing patterns of electron distribution that don’t always follow the electron filling rules. Predicting how they will form ions is also not always obvious.Thus, you should refer to the common ion periodic table to determine the ionic states of transition and inner transition elements (Figure 4.7).
Figure 4.7 Common Ionic States of the Elements. For elements that have more than one common ionic state, both states are listed. Note that when mercury carries a +1 charge, it forms an uncommon polyatomic ionic state, Hg22+ where two Hg atoms share electrons and then each also have a +1 charge state (see section XX for more details about polyatomic ions and Hg22+). For the printable PDF version of this table (with the common polyatomic ions), click the link below:
(Back to the Top)
4.3 Ionic Bonding
Most of the rocks and minerals that make up the Earth’s crust are composed of positive and negative ions held together by ionic bonding. An ionic compound is an electrically neutral compound consisting of positive and negative ions. You are very familiar with some ionic compounds such as sodium chloride (NaCl). A sodium chloride crystal consists of equal numbers of positive sodium ions (Na+) and negative chloride ions (Cl−).
Anions and cations have opposing charges. Because of this, they are attracted to one another. When an anion and a cation are drawn together due to this electrostatic attraction, they can form an ionic bond. This kind of bond is the result of opposing charges attracting one another, and is distinct from other types of bonding. Two or more ions bound by electrostatic attraction make an ionic compound. The simplest ionic compounds are binary ionic compounds or those that only contain two atoms, one acting as the cation, and one acting as the anion. Thus, we will focus on the formation of binary ionic compounds first.
Sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. The chloride ion has one excess electron, giving it a -1 charge. The result of this electron transfer is that the sodium cation and chloride anion become bound through electrostatic attraction, forming sodium chloride, an ionic compound. Note that, electrons cannot be simply “lost” to nowhere in particular, they always end up going to another atom or molecule. Ionic reactions can be represented by electron dot diagrams, as shown below for sodium chloride.
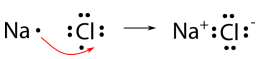
The ionic bond is the attraction of the Na+ ion for the Cl− ion. It is conventional to show the cation without dots around the symbol to emphasize that the original energy level that contained the valence electron is now empty. The anion is now shown with a complete octet of electrons. The final formula for sodium chloride is NaCl. Notice that both ions are represented but their charges are not shown. This is because within ionic compounds the overall charge on the compound is zero, i.e. the charge states of the cation(s) and the anion(s) involved in the bond need to be paired in such a way that the number of positive charges equals the number of negative charges. For sodium chloride this is an easy task as one chloride ion has a -1 charge and one sodium ion has a positive charge +1, cancelling each other to zero. Also note that in chemical formulas that the cation always comes first and the anion is always placed second in the formula.
For a compound such as magnesium chloride, it is not quite as simple. Because magnesium has two valence electrons, it needs to lose both to achieve the noble-gas configuration. Therefore, two chlorine atoms will be needed.
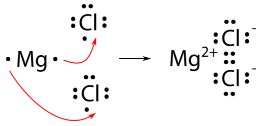
The final formula for magnesium chloride is MgCl2. Note that the subscript (2) next to the chloride ion, indicates that there are two chloride ions paired with each magnesium cation. When there is only one ion present in a formula, (i.e the magnesium ion in this case), the subscript of one is implied instead of shown in the formula. As in the case of NaCl, there are no charges shown in the final formula of MgCl2. This is because the positive charge of the magnesium ion (+2) is balanced by the negative charge of the two chloride ions [2 X (-1) = -2] giving the overall molecule a net charge of zero.
(Back to the Top)
4.4 Practice Writing Correct Ionic Formulas
To predict and write correct chemical formulas, the key fundamental steps that are required, are (1) knowing the charge states of the ions and (2) using basic math to help you determine how many cations and anions are needed to reach a zero charge state, (3) writing the chemical forumulas with the cation first followed by the anion, and (4) writing the formula with the lowest ratio of cations and anions to create a net neutral compound.
Overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an overall neutral net charge. Of note, ionic bonds usually occur between a metal and a nonmetal. This will help you recognize ionic compounds more easily, once we learn about covalent bonding (which occurs most commonly between two nonmetals, or between a nonmetal and a semimetal (metalloid).
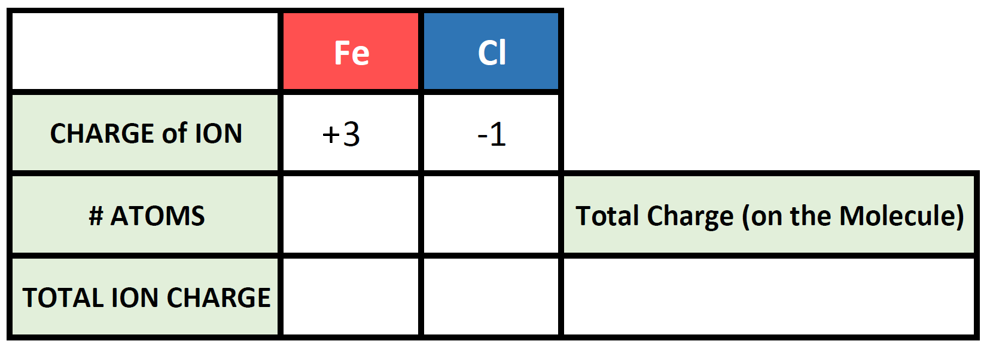
So, say we want to write the correct chemical formula for a molecule that contains Fe3+ as the cation, and Cl– as the anion. What is the correct ionic formula?
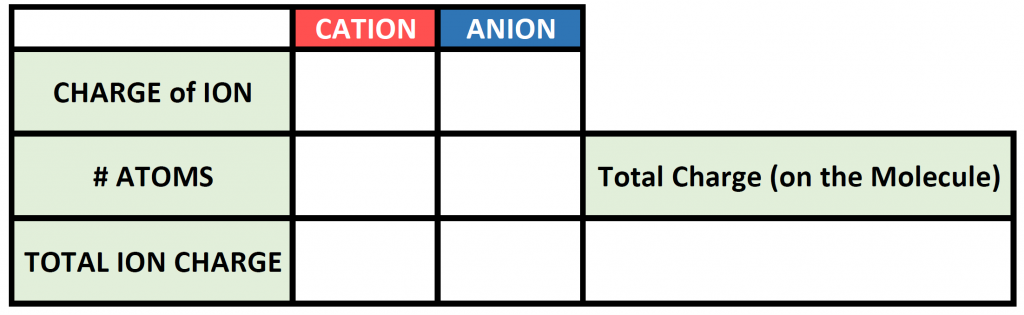
To begin this type of problem, I recommend drawing out a charge box or a charge table to help you keep track of the number of ions used, the charges of those ions, and the overall positive and negative charges on the molecule. Drawing out the electron dot symbols can also be helpful. Here is an example of a generic charge box
Let’s try it out for our example of Fe3+ and Cl–. First, let’s fill in what we know about each element and it’s ionic state:
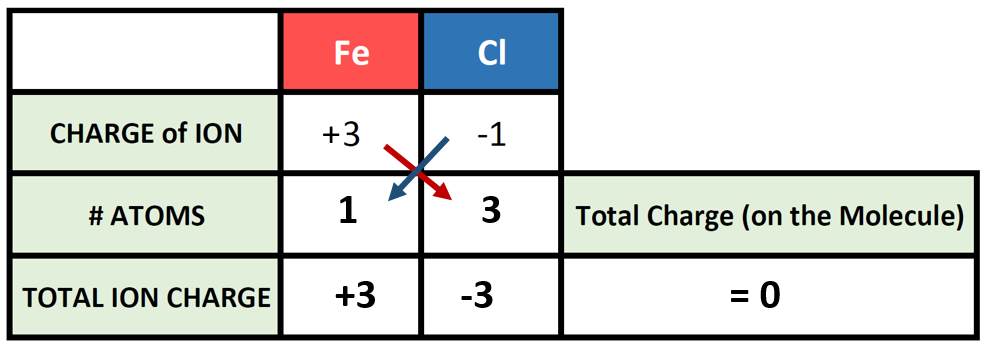
So now we have our charge box set up with our known information. Now we need to figure out how many atoms of the cation and the anion are required to cancel out the overall positive and negative charge on the resulting molecule. To do this, it is often useful to use the cross-multiplication strategy, where you try using the charge number for the cations, as the number of atoms of anion required, and the charge number for the anion as the number of atoms of the cation required. Multiply each of the ion charges by the number of atoms to calculate the total ion charges of the cation(s) and anion(s) present and then add these numbers together to find the total charge on the compound. This will usually get you to the stable ionic formula that has a net neutral charge of zero.
The # of atoms column then becomes the subscripts that you need to use to construct the correct ionic formula. In this case 1 atom of iron (Fe) with 3 atoms of chlorine (Cl) for a formula of FeCl3.
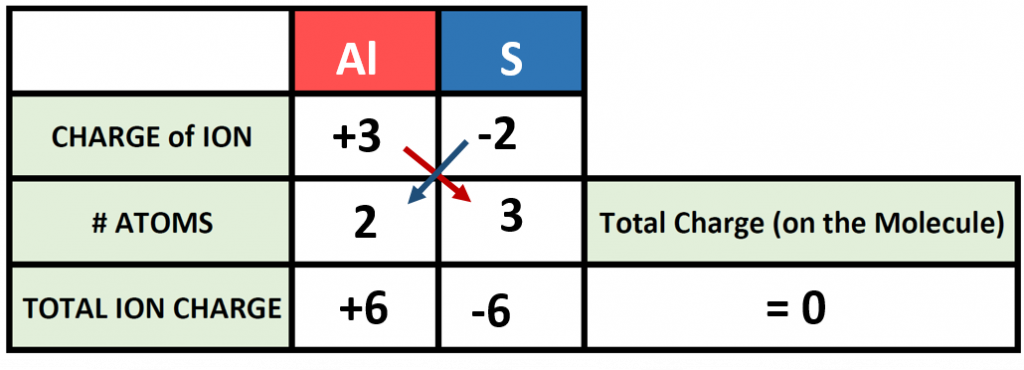
The previous example is pretty straight forward, and you may have been able to construct the formula in you head. However, as the complexity of formula making increases, it is good to be able to use the charge box method to double check your work. For example, what would the correct ionic formula be for aluminum sulfide? First, identify the two atoms involved (Aluminum and Sulfur) and start building your charge box with what you know from the periodic table. From the periodic table in Figure 4.7, you can see that aluminum forms a cation with a +3 charge whereas sulfur forms an anion with a -2 charge state.
For step 1: Add in the correct charge for the cation and anion in question, in this case +3 for Al and -2 for S. For Step 2: Use the cross multiply rule to predict how many atoms will be needed from each type and multiply through the total ion charge for both the cation and anion. For Step 3: Add the products together to be sure that your compound is stable and the net charge on the formula is zero. Step 4: Use the # Atoms value to create the subscripts for your chemical formula. In our example, we require 2 atoms of Al and 3 atoms of S. This would be written as Al2S3 as the final product.
(Back to the Top)
4.5 Naming Ions and Ionic Compounds
Some compounds have common names, like water for H2O. However, there are thousands of other compounds that are uncommon or have multiple names. Also, the common name is usually not recognized internationally. What looks like water to you might look like agua or vatten to someone else. To allow chemists to communicate without confusion, there are naming conventions to determine the systematic name of a chemical. For the chemistry naming system in this text, we will primarily be using the International Union of Pure and Applied Chemistry (IUPAC) naming system. Note that there is also an older and more archaic (-ous and -ic) naming system, in addition to the IUPAC system. In some instances the older naming system is still in high use. These deviations from the IUPAC system will be noted throughout the text, as you will likely still see this older nomenclature still in use within chemical laboratories and the health sciences field.
The convention for naming cations is very easy. It is simply to take the element name and add the term ‘ion’ to the end of it. So if we are referring to a sodium atom that has lost one electron (Na+), we would use the term sodium ion. This indicates that sodium is in the +1 charge state, rather than the elemental form of sodium (which has an equal number of protons and electrons and is neutral in charge). Using the ion naming system when referring to ions, rather than the elemental names of atoms is important, as the reactivity of the ion vs the elemental form of a substance can be quite different. For example, if you add the sodium ion to your glass of drinking water in the form of NaCl (or table salt), you will have a nice salty drink on your hands. On the other hand, if you add the elemental form of sodium to your glass of drinking water, it will explode in your face, as the elemental form of sodium is very reactive with water!
For cations that have more than one charge state the name of the atom is followed by a roman numeral and then the term ion, to distinguish the different ionic states. For example, iron has two predominant ionic forms, Fe2+ and Fe3+. Thus, in naming these two ions, we would refer to the first one as the iron (II) ion, and the second as the iron (III) ion. This way, there is no confusion about which ion is being referred to when discussing a compound.
Naming anions is a little more complicated. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. For example, Cl– is referred to as the chloride ion, rather than the chlorine ion. In this case, the ‘-ine’ ending of chlorine is dropped and replaced with the ‘ide’ ending. For sufur, the ‘-ur’ ending is dropped and replaced with ‘ide’ to form the sulfide ion. Similarly phosphorus is converted to the phosphide ion, nitrogen to the nitride ion, and oxygen to the oxide ion. The ‘-ide’ ending is useful because it helps the listener distinguish very quickly between the different types of ions being discussed (the cation which retains the element name vs. the anion which changes the elemental name to the ‘-ide’ ending).
When naming ionic compounds the term ion is dropped and the cation and anion names are placed together, with the cation always listed first and the anion listed last. If the elements involved in the ionic bond only have one possible ionic state, no roman numerals are needed in the name. For example, when the Na+ and the Cl– come together to make NaCl, the resulting compound is called sodium chloride. Similary, if Mg2+ and Cl– come together to make MgCl2, the resulting compound is called magnesium chloride. However, if the elements involved in the ionic bond have more than one possible ionic state, the roman numeral system is used to clarify which ion is participating in the bond. For example, if Fe3+ and Cl– come together to form FeCl3, we will need to distinguish it from Fe2+ coming together with Cl– to form FeCl2 in the name so that everyone will understand which ion of iron is being referred to in the reaction. In this case, the first compound will be called iron (III) chloride, and the second compound is iron (II) chloride.
The key feature about naming ionic compounds is that you should be able to draw the structure from the name, and that you should be able to create the name from the structure. Let’s do some practice!
(Back to the Top)
4.6 Polyatomic Ions
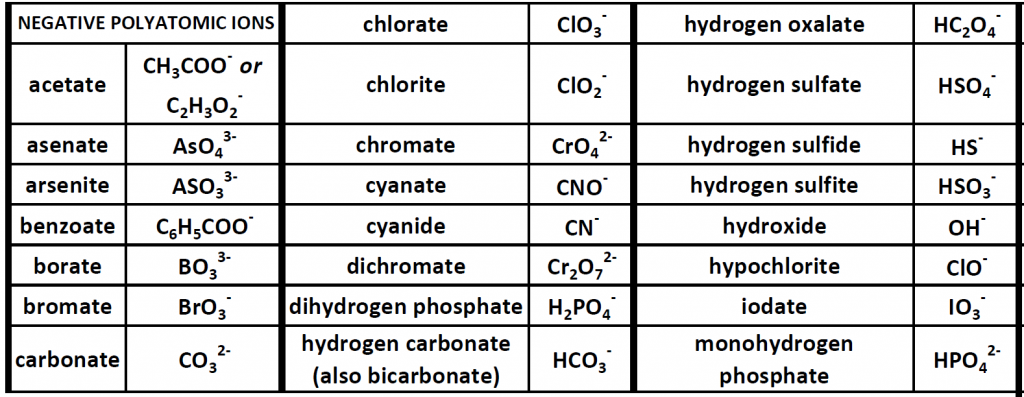
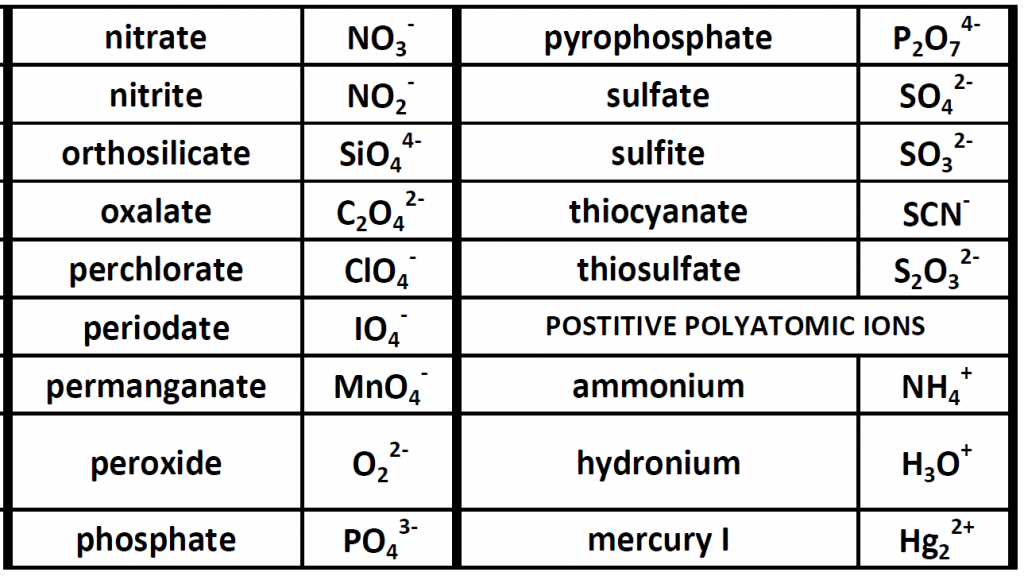
Up until now, we have been looking at compounds involving monoatomic ions, or ions that occur with a single atom. However, many commonly found ions are composed of multiple atoms that are bound to one another through the sharing of electrons, or covalently. These ions behave as a single unit, bearing a charge and interacting with other ions and compounds just like the monatomic ions discussed above. Because these ions are made of multiple atoms, they are called polyatomic ions. It is more common for polyatomic ions to be negatively charged than to be positively charged. Below is a chart showing some commonly encountered polyatomic ions.
Table 4.1 Common Polyatomic Ions
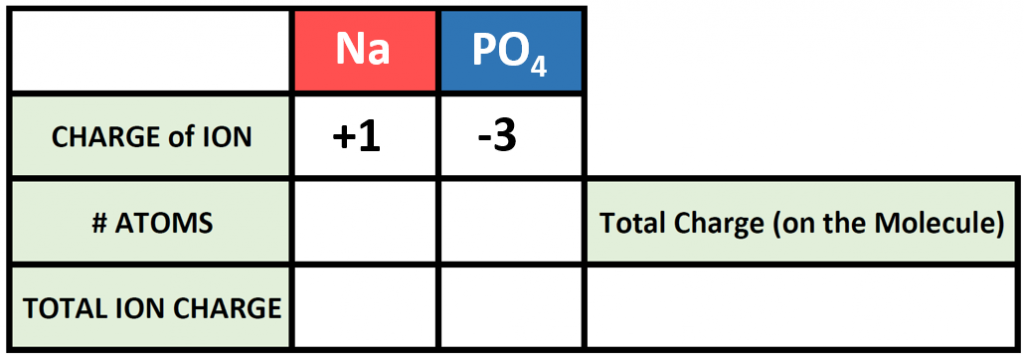
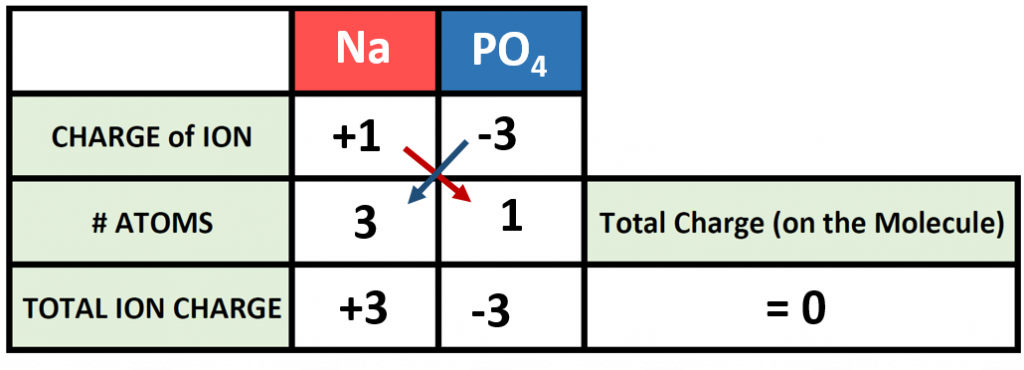
Polyatomic ions can be thought of in a very similar way to monoatomic ions, in that they are ionized by either gaining or losing electrons so that they carry a charge. If they gain electrons, they will become an anion and carry a negative charge, and if they lose electrons, they will become a cation and carry a positive charge. The charge of a polyatomic ion is represented as a supercript that is placed at the upper righthand edge of the ion. For example, for the phosphate ion, the chemical formula is PO43-. This indicates that the overall -3 charge is distributed to the entire PO4 molecule, and that when it is involved in forming an ionic compound, the entire PO43- ion moves as and is treated as a single unit. Let’s try making a few compounds using phosphate as an example. First let’s build a molecule of sodium phosphate. Note that when you are asked to build molecules from their name, you can often recognize when you have a polyatomic ion due to the name. Recall that monoatomic anions end in the suffix ‘-ide’. Thus, when you see a different suffix ending, such as ‘-ate’ or ‘-ite’, this should indicate that you are dealing with a polyatomic ion and you should refer to the table above to help you discern the correct ion formula to use. For the sodium phosphate example, we can build this molecule using the same charge box diagram that we used above to construct the simpler biatomic structures above. First we need to place the ions and their charge states into the table. In this case, we know that sodium is a cation with a +1 charge and the phosphate ion is an anion with a -3 charge.
Note that in our table, we are treating the polyatomic ion as a single unit. We can then continue to use our cross multiplication strategy to determine how many cations and anions are needed to create an overall molecule that is neutral in charge.
Thus, we will need 3 atoms of sodium and one molecule of phosphate to complete our structure. Overall the chemical formula of sodium phosphate is written as Na3PO4. Note that the naming of the resulting molecule is done in exactly the same way as with other ionic compounds. The name of the cation comes first (using roman numerals when necessary) followed by the name of the anion (in this case phosphate).
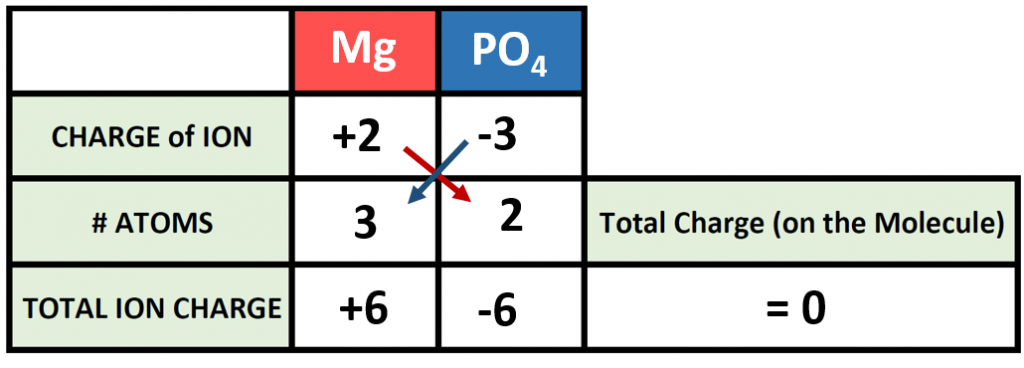
How about a more complicated example? How would we make a molecule of magnesium phosphate? Start building your molecule using the charge box diagram, noting this time that magnesium forms and Mg2+ ion.
Setting up the charge box for this compound is not more difficult than any other compound. However, one must be careful when writing out compounds that require more than one polyatomic ion within the chemical formula. In this case we need 2 phosphate ions to combine with 3 magnesium ions to form magnesium phosphate. The cation in this case is written the same, however, parentheses are needed when expressing the 2 phosphate ions, as follows:
Mg3(PO4)2
The parentheses around the phosphate ion ensure that it is clear that you need two entire PO43- ions within this complex. A structural diagram of what this molecule would look like is shown below. Note that each straight line is being used here to indicate a covalent bond within the phosphate ion. Each straight line represents two electrons (or an electron pair) that is being shared between the atoms. Covalent bonding will be described in more detail in chapter 4. For now, it is important to remember that the polyatomic ions move together as a single unit because the atoms that are sharing electrons must stay in close proximity with one another. The ionic bonds are indicated with the (+) and (-) symbols. For magnesium phosphate there are a total of 6 ionic bonds that are formed.
Another strange example is mercury (I) chloride. This one is an exception to our normal bonding rules. You would predict based on charge possibilities that mercury (I) chloride should have the chemical formula of HgCl, as the chloride ion has a charge of -1, and mercury (I) is indicated to have a charge of +1. However, in this unique case, this formula is incorrect. Mercury is unusual in that its singly ionized oxidation state, mercury(I), is found as a dimeric cation, Hg22+, where two atoms of mercury are actually covalently bonded to one another as a polyatomic ion. Each mercury atom within the bonded pair has a charge state of +1. This give the overall ion a +2 state, as shown below:
Unfortunately, this polyatomic ion does not have a unique name that distinguishes it from normal monoatomic cations. Thus, you will need to remember this unique member. The final mercury (I) chloride chemical formula needs 2 chloride ions to complete the structure, for a minimal chemical formula of Hg2Cl2.
While mercury (I) chloride is rarely found in nature, during the 18th and 19th centuries, known as calomel, it was commonly used as medicine to treat infectious diseases like syphilis and yellow fever. It was also used as a general tonic to make patients regurgitate and release their body from ‘impurities’. Calomel had extreme side effects and toxicity during its medical use causing both loss of hair and teeth. In fact, calomel was also a common ingredient in teething powders in Britain up until 1954, causing widespread mercury poisoning in the form of pink disease, which at the time had a mortality rate of 1 in 10. Once the cause of pink disease was linked with mercury toxicity, the substance was removed from these powders. In the United States, its use faded in the late 1800’s with the discovery of more effective treatments, such as the discovery of penicillin in the late 19th century by Alexander Flemming.
Abraham Lincoln and “Blue Mass”
“Blue mass,” a medication that consisted of elemental mercury with various additives, was commonly used for all kinds of complaints in the Civil War-era United States. Though mercury was a known toxin, it was a prominent feature in medical treatment for “hypochondriasis,” a condition that may have included various problems we now understand as mood disorders, along with digestive system issues. Abraham Lincoln was known to exhibit the symptoms of hypochondriasis, and he took the blue mass medication. Interestingly, he was known by friends and acquaintances to suffer from insomnia and erratic mood, and there is some evidence that he displayed additional neurological abnormalities. These are symptoms of mercury poisoning. Within the body, elemental mercury, which is uncharged, is oxidized to its mercuric form (Hg2+), which has a +2 charge. This form of mercury is devastating to many body systems, causing dysfunction that may have been responsible for Abraham Lincoln’s symptoms. His treatment may have been more harmful than the problems for which it was intended, due to medicine’s lack of understanding.
(Back to the Top)
4.7 Naming Polyatomic Ions
Polyatomic ions have special names as noted in Table 4.1. Many of them contain oxygen and are called oxyanions. When only one oxyanion for an element exists, the ending of the primary element is given the ‘-ate’ ending. For example, the oxyanion of carbon is called carbonate (CO32-). However, when different oxyanions exist using the same element but have a different number of oxygen atoms, prefixes and suffixes are used to tell them apart. For example, if two oxyanions exist, the one with the lower number of oxygens will be given the ‘-ite’ ending and the one with more oxygens will be given the ‘-ate’ ending. Oxyanions of nitrogen and sulfur are a good example:
NO2– is called Nitrite
NO3– is called Nitrate
SO32- is called Sulfite
SO42- is called Sulfate
Sometimes there may be three or four oxyanions. In this case, the prefix ‘hypo-‘ will be used to indicate one less oxygen than ‘-ite’ form. When four oxyaions exist there is also a ‘per-‘ prefix, meaning one more oxygen that the ‘-ate’ form. The chlorine family of ions is an excellent example where these prefixes are needed.
ClO– is called hypochlorite
ClO2– is called chlorite
ClO3– is called chlorate
ClO4– is called perchlorate
Occasionally, you will see a bi– prefix. This is an older prefix, it means the compound can both take up and lose a proton (H+). IUPAC nomenclature will use hydrogen in the name, whereas the older nomenclature uses the bi-prefix. In either case, the oxyanion will have a hydrogen in it, decreasing its charge by one. For instance, there is carbonate (CO32-) and hydrogen carbonate (HCO3–). You may also see hydrogen carbonate referred to as bicarbonate.
One last prefix you may find is thio-. It means an oxygen has been replaced with a sulfur within the oxyanion. Cyanate is OCN–, and thiocyanate is SCN–.
Naming ionic compounds that contain polyatomic ions is done in exactly the same way as with other binary ionic compounds. The name of the cation comes first (using roman numerals when necessary) followed by the name of the anion. Refer to Table 4.1 to determine the correct names for the polyatomic ions.
(Back to the Top)
4.8 Properties and Types of Ionic Compounds
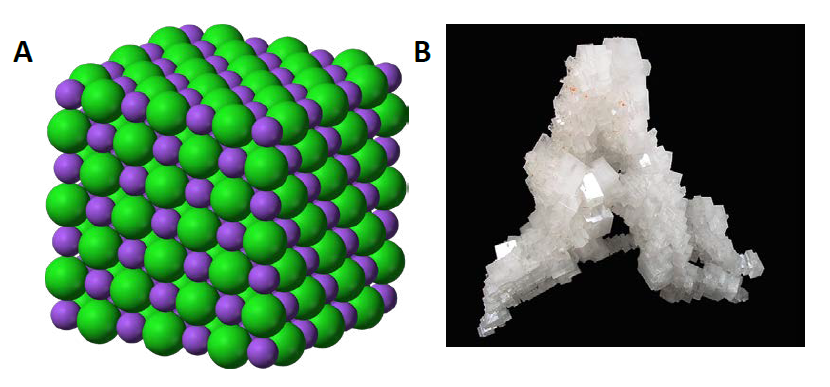
Ionic compounds are held together by the electrostatic forces created by the attraction of the positively charged cations and negatively charged anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH4+) and carbonate (CO32-) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbors, so are not considered to be part of individual molecules, but instead as part of a continuous three-dimensional network or lattice, usually in a crystalline structure. Figure 4.8 shows the structure of sodium chloride (NaCl)
Figure 4.8 Crystal Lattice. (A) The crystal structure of sodium chloride, NaCl, a typical ionic compound. The purple spheres represent sodium cations, Na+, and the green spheres represent chloride anions, Cl−. (B) Halite, the mineral form of sodium chloride, forms when salty water evaportates leaving the ions behind.
Source: (A) Benjah-bmm27 (2010). (B) Lavisky, R. (2010) Both (A) and (B) Available at: https://en.wikipedia.org/wiki/Ionic_compound
Ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing hydroxide (OH−) or oxide (O2−) ions are classified as bases. All other ionic compounds without these ions are known as salts. Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids, they are most often electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized. When the ions are mobilized in solution, they are referred to as electrolytes, due to their ability to conduct electricity.
(Back to the Top)
4.9 Arrhenius Acids and Bases
H+ and OH– ions are the key players in acid-base chemistry, under the Arrhenius definitions for acids and bases. Arrhenius defined an acid as a compound that increases the concentration of hydrogen cations (H+) in aqueous solution. Many acids are simple compounds that release a hydrogen cation into solution when they dissolve and can be recognized as ionic compounds that contain H+ as the cation. Similarly, Arrhenius defined a base as a compound that increases the concentration of hydroxide ions (OH−) in aqueous solution. Many bases are ionic compounds that have the hydroxide ion as their anion, which is released when the base dissolves in water.
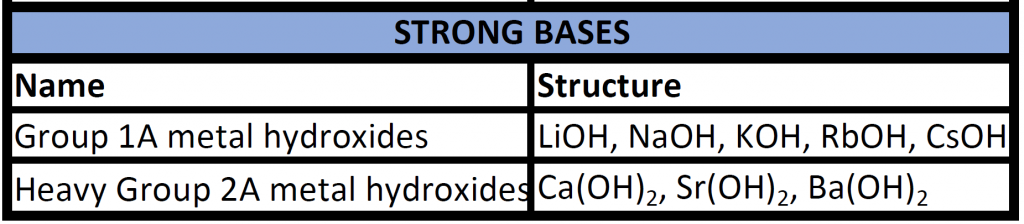
Arrhenius bases are named according to standard ionic nomenclature, with the strongest bases being the hydroxides of the alkali metals and the heavier alkaline earth metals. You will be expected to recognize strong bases.
Arrhenius acids have a nomenclature system that is a little more complex, since their structures can include both binary compounds as well as polyatomic anions. In naming acids from binary compounds, the prefix ‘hydro-‘ is used to represent the cation H+, and the suffix ‘-ic’ acid is used to indicate that it is an acidic form. The element name of the anion can be used directly, as is the case for H2S known as hydrosulfuric acid, or more commonly, the anion is modified by dropping the ‘-ine’, ‘-ous’ or ‘-ogen’ ending before replacing with the suffix ‘-ic acid’, as is the case for HCl which is known as hydrochloric acid, H3P which is known as hydrophosphoric acid and H3N which is known as hydronitric acid.
If an acid contains a polyatomic ion, no leading prefix is used to indicate the H+ cation. This is implied within the name. For polyatomic anions ending with the suffix ‘-ate’, the acid is named as the [anion name] + the ‘-ic acid’ suffix. For example, when the sulfate ion (SO42-) is complexed with H+ as the cation, the overall formula will be H2SO4 and the resulting acid will be named sulfuric acid. Dropping the prefix distinguishes polyatomic acids from the binary acids, in this case sulfuric acid (H2SO4) is distinguished from hydrosulfuric acid (H2S). If a polyatomic anion has the ‘-ite’ ending, the acid name will be written as the [anion name] + the ‘-ous acid’ suffix. For example HNO2 would be nitrous acid, and HNO3 would be nitric acid. The prefixes ‘hypo-‘ and ‘per-‘ are also retained in the acid nomenclature for elements that have many oxyanion states. For example the chlorine containing oxyanions can form the following acids:
HClO = hypochlorous acid
HClO2 = chlorous acid
HClO3 = chloric acid
HClO4 = perchloric acid
These are all distinguished from the binary chlorine-containing acid:
HCl = hydrochloric acid
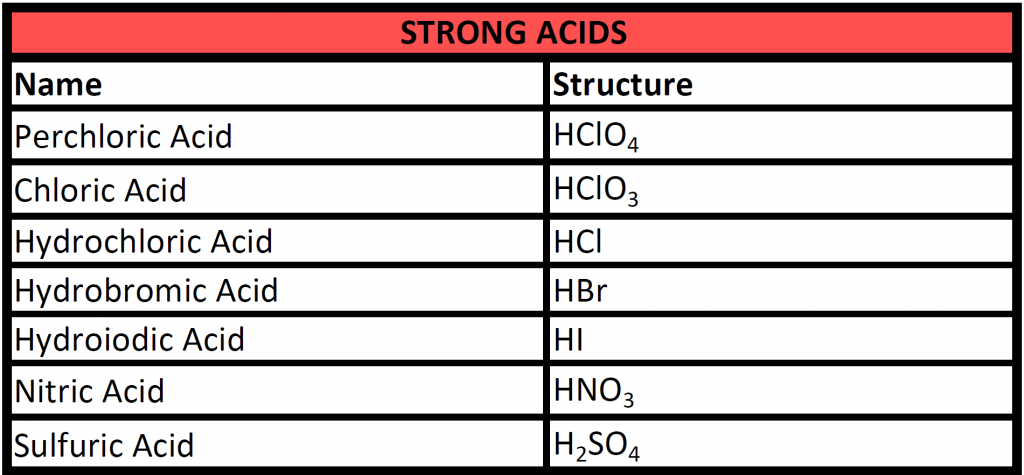
Strong acids are ones that completely dissociate into their ionic forms in solution. The following table lists common strong acids that you will need to be familiar with.
Quiz Yourself: More Practice Naming Compounds
(Back to the Top)
4.10 Ions, Neurons and Actions Potentials
Within biological systems, ions play many important roles including muscle contraction, cellular metabolism, and ATP energy production, but perhaps one of the most important roles is in the function of the brain. The brain is an extremely complex organ that is composed of trillions of tiny cells called neurons. A neuron, also known as a nerve cell, is an electrically excitable cell that receives, processes, and transmits information through electrical and chemical signals. These signals between neurons occur via specialized connections called synapses. Neurons can connect to each other to form neural pathways, and neural circuits that can be quite complicated. Neurons are the primary components of the central nervous system, which includes the brain and spinal cord, and of the peripheral nervous system, which comprises the autonomic nervous system and the somatic nervous system.
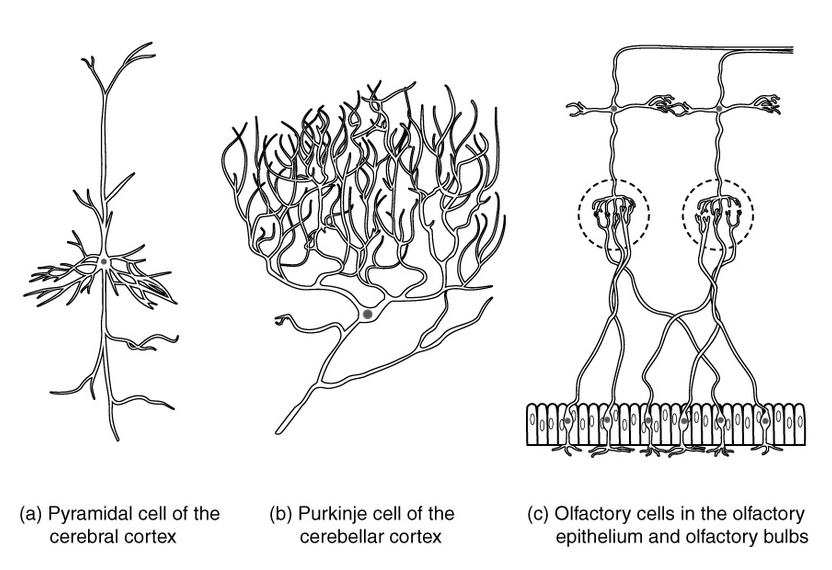
There are many types of specialized neurons that can be classified by shape, location, or function (Fig. 4.9). Sensory neurons respond to one particular type of stimulus such as touch, sound, or light and all other stimuli affecting the cells of the sensory organs, and converts it into an electrical signal via transduction, which is then sent to the spinal cord or brain. Motor neurons receive signals from the brain and spinal cord to control everything from muscle contractions to glandular output. Interneurons connect neurons to other neurons within the same region of the brain or spinal cord in neural networks.
Figure 4.9 Examples of Different Types of Neurons. (a) Pyramidal neurons found in the cerebral cortex is a multipolar cell with a cell body that is shaped somewhat like a pyramid. (b) The Purkinje cell in the cerebellum was named after the scientist that originally described it. (c) Olfactory neurons are named for their function in the sense of smell.
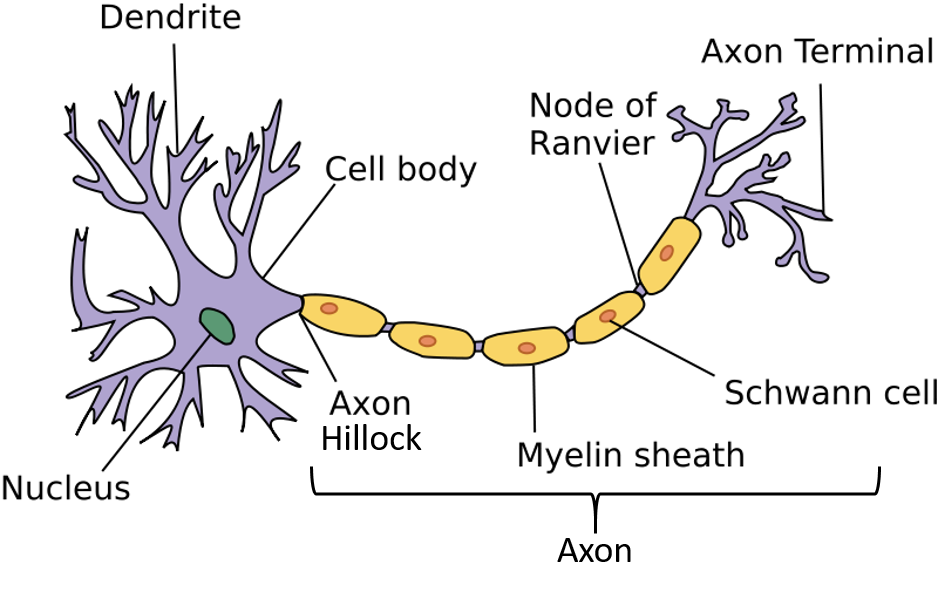
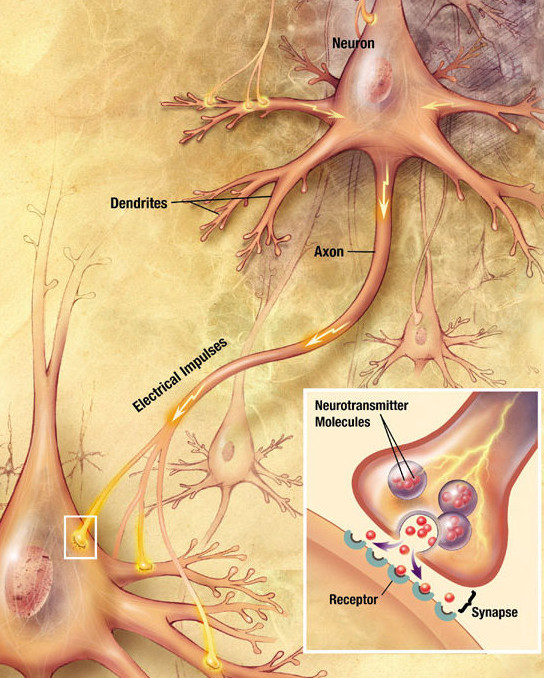
A typical neuron consists of a cell body (soma), dendrites, and an axon (Fig 4.10) The term neurite is used to describe either a dendrite or an axon, particularly in its undifferentiated stage. Dendrites are thin structures that arise from the cell body, often extending for hundreds of micrometers and branching multiple times, giving rise to a complex “dendritic tree”. An axon (also called a nerve fiber) is a special cellular extension (process) that arises from the cell body at a site called the axon hillock and travels for a distance, as far as 1 meter in humans or even more in other species. Most neurons receive signals via the dendrites and send out signals down the axon. For an action potential to be sent down the axon, a threshold signal must be received by the dendrites and transmitted to the axon hillock. If the signal is strong enough when it reaches the axon hillock, a single all or nothing action potential will be sent down the axon causing the release of neurotransmitters into the synaptic cleft, as depicted in Figure 4.11.
Figure 4.10 Anatomy of a Neuron. Depicted within the diagram is the neuron with the central cell body (soma) and typical dendrite and axon projections. The dendrites of a neuron are typically where outside signals are received and the axon is the used to transmit the chemical signal to downstream target cells in the communication pathway. Figure adapted from Wikimedia
Figure 4.11 Neuronal Signaling. This diagram depicts the cell-cell communication between neurons after the firing of an action potential down the axon of a pre-synaptic neuron. At the axon terminal, secretory vesicles containing neurotransmitters such as serotonin and dopamine, are released into the synaptic cleft where they can can interact with receptors on the dendrite or soma of the post-synaptic neuron. Figure provided by Wikimedia.
Numerous axons are often bundled into fascicles that make up the nerves in the peripheral nervous system (like strands of wire make up cables). Bundles of axons in the central nervous system are called tracts. The cell body of a neuron frequently gives rise to multiple dendrites, but never to more than one axon, although the axon may branch hundreds of times before it terminates. At the majority of synapses, signals are sent from the axon of one neuron to a dendrite of another. There are, however, many exceptions to these rules: for example, neurons can lack dendrites, or have no axon, and synapses can connect an axon to another axon or a dendrite to another dendrite.
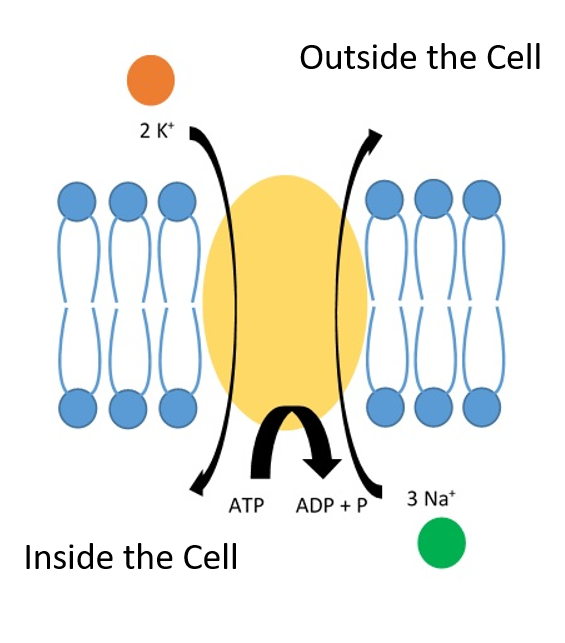
To become an excitable cell that can transmit an electric signal, neurons generate a negative resting potential within their cells. This is accomplished by sequestering cations and anions in different concentrations either inside the cell (intracellular) or outside the cell (extracellular). This leaves the inside of the neuron with a resting potential of -70 mV. Thus, the cell is said to be polarized or negatively charged. The neuron is able to generate this negative resting potential largely through the use of the Sodium (Na+)/Potassium (K+) ATPase Protein. This protein is embedded in the plasma membrane of all neurons, where it functions as a pump. Protein pumps use energy to physically pump molecules across the plasma membrane against their concentration gradient. The energy being used for this process is Adenosine Triphosphate (ATP). In this case, 3 sodium ions are pumped out of the cell, while 2 potassium ions are pumped inside the cell for each ATP molecule that is broken down into Adenosine Diphosphate (ADP) (Figure 4.12). This creates an electrochemical gradient where a high concentration of sodium ions are outside of the cell and a high concentration of potassium ions are inside the cell. Similarly, calcium ions accumulate on the outside of the neuron. The inside of the cell also contains numerous organic anions and phosphate anions causing the large negative resting potential of -70 mV within the cell.
The Na+/K+ ATPase pump is widely expressed in all neurons and is constantly working to maintain this gradient. In fact, this is one of the major uses of energy within the body consuming almost 20% of the body’s total energy each day. The formation of this concentration gradient makes it possible for neurons to send electrical impulses down the cell axon and communicate with downstream target cells. This makes it possible to think, move our muscles, and sense the outside world through touch, sight, hearing and smell.
Figure 4.12. The Sodium-Potassium ATPase Pump. The Na+/K+ ATPase Pump uses the energy of ATP to pump 3 Na+ out the cell and 2 K+ into the cell. This gradient enables neurons to maintain a resting electrical potential of -70 mV within the cell.
Image from Wikimedia Commons
To generate an action potential, a neuron needs a ripple of positive current to flow down the axon. When it reaches the terminal of the axon, this is a signal that neurotransmitters, such as dopamine, serotonin or glutamate should be released into the synaptic cleft (Fig. 4.11) These small chemical messengers are used to communicate with the downstream neuron or muscle cell. Within the neuron, ion channels play an important role in generating the action potential. Ion channels are proteins embedded within the plasma membrane of the neuron that form a pore large enough for specific ions to pass through. Ion channels do not use energy and can only allow the ions to flow down their concentration gradient from an area of higher concentration to an area of lower concentration through a process called facilitated diffusion.
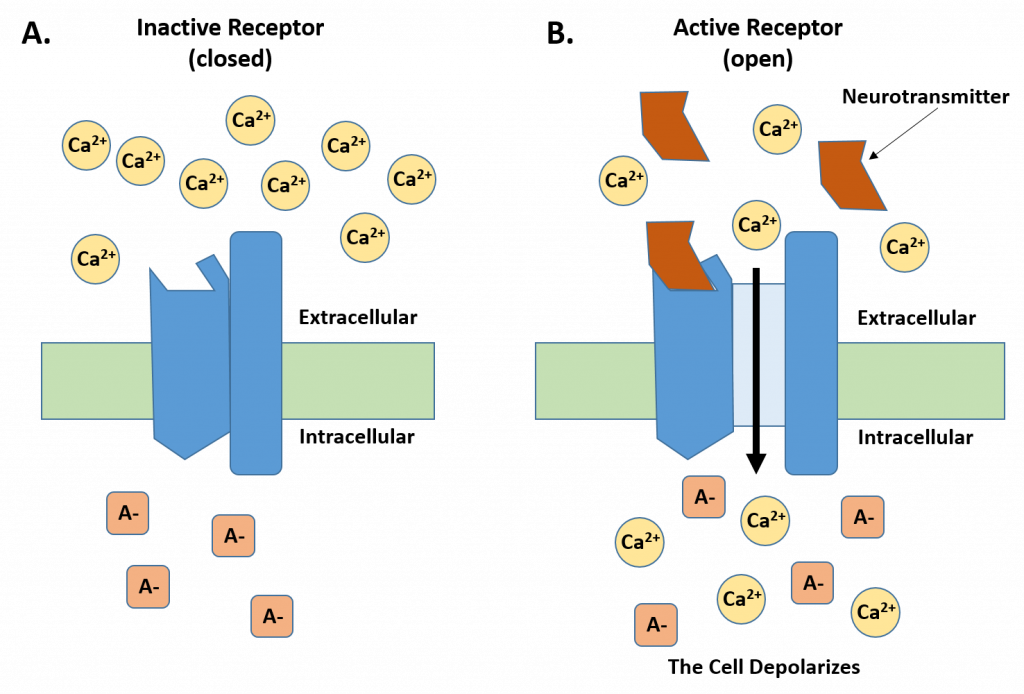
Two types of ion channels that are important for generating an action potential are receptor/ligand activated channels and voltage-gated channels. For the receptor/ligand activated channel, a small molecule that is acting as a chemical messenger (also called a ligand), binds to the receptor and causes a conformational change that opens the ion channel (Fig. 4.13). Usually these types of receptors are found on the dendrite of the receiving neuron, where they will bind to neurotransmitters. Once bound to a neurotransmitter, the receptor opens a calcium channel, allowing calcium ions to flow quickly into the cell making the local area become less negative inside. When the charge inside the neuron becomes closer to zero (or more neutral), this is called a depolarization event.
Figure 4.13. Receptor/Ligand Activated Channels. (A) Shows a receptor in the closed conformation during the resting state of the neuron. Note that calcium ions are in high concentration outside of the cell while increased anion concentrations inside the cell create a -70 mV resting state. (B) When neurotransmitters are released from the axon into the synapse, they will bind with the receptor causing a conformational change in the receptor that opens the calcium ion channel. Ca2+ flows into the cell down its concentration gradient causing localized depolarization within the cell.
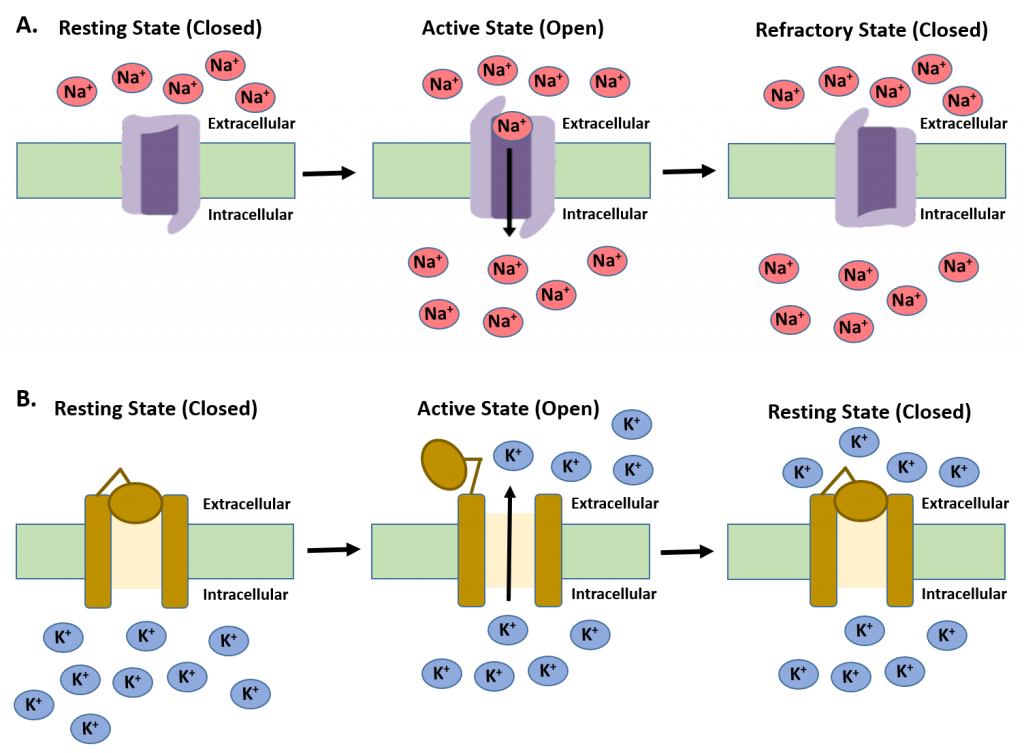
This localized depolarization event, if strong enough, can activate neighboring voltage-gated sodium channels (Fig. 4.14A). These channels are sensitive to the charge state in the neuron and will open when the charge is reduced in the local area. At this time, the voltage-gated sodium channels will undergo a structural change that allows sodium ions to rapidly flow into the cell, down their concentration gradient. This will further cause the neuron to depolarize and cause the opening of other voltage-gated sodium channels that are in close proximity. When the charge state of the neuron surpasses neutral and becomes positive on the inside (approximately +30 mV), the voltage-gated sodium channels undergo another conformational change, closing the channels and stopping the inflow of sodium ions. At this point, the voltage-gated potassium channels are activated allowing potassium ions the flow out of the cell down their concentration gradient (Fig. 4.14B).
Figure 4.14 Voltage-Gated Ion Channels. (A) The Voltage-Gated Sodium Channels are closed when the neuron is in the resting state. Following neurotransmitter stimulation, the cellular depolarization causes the voltage-gated sodium channels to undergo a conformational change, opening the sodium channel and allowing the influx of Na+ into the cell. When the cellular charge becomes positive (approximately +30 mV) the voltage-gated Na+ channels close due to an additional conformation change and enters a refractory period where it cannot be reactivated. The lowering of the charge state within the neuron to -70 mV restores the protein conformation to the resting state. (B) The voltage-gated potassium channel is closed during the resting state of the neuron and is not activated until the polarity of the cell shifts to approximately +30 mV. Following activation, potassium ions move out of the cell, down their concentration gradient and restore the resting potential of the neuron to -70 mV.
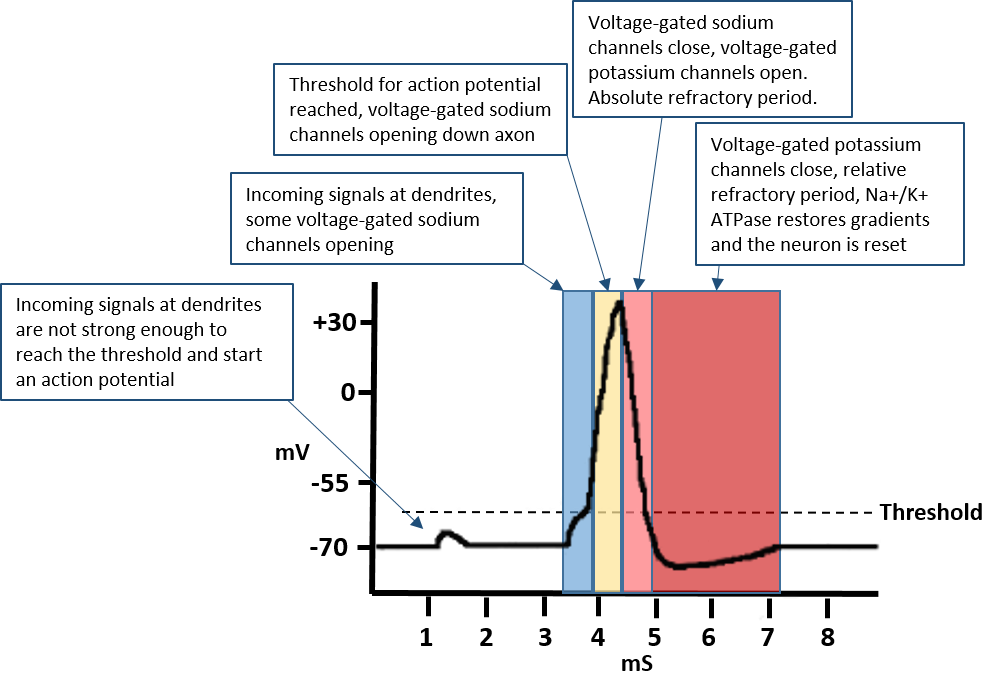
The opening of the voltage-gated potassium channels restores the negative resting potential of the neuron. However, the ion gradients are out of balance at this time period with high levels of sodium inside the cell and high levels of potassium outside of the cell. This creates an absolute refractory period where the neuron cannot be reactivated. Once the potassium channels have closed and the negative potential of the neuron has been restored, the neuron enters a relative refractory period where a new action potential is inhibited but not impossible to elicit. During this time, the Na+/K+ ATPase pump restores the ion gradient, such that sodium is pumped out of the cell, and potassium is pumped back into the cell. At this point, the neuron is reset and is fully sensitive to receiving another signal. This can be represented graphically in terms of voltage over time, where the action potential depolarization event can be correlated with the opening and closing of the specific ion channels (Fig 4.15) The entire action potential can be accomplished within 4-5 milliseconds (mS).
Figure 4.15 Graphic Representation of an Action Potential. The resting state potential of a neuron is -70 mV. An action potential is propagated down an axon when a threshold of -55 mV reaches the axon hillock. This causes the opening of voltage-gated sodium channels along the axon and the release of neurotransmitter from the axon terminal. The voltage-gated sodium channels close and become refractory when the cell potential reaction +30 mV. This also results in the opening of the voltage-gated potassium channels which re-establish the resting state potential of the neuron. The Na+/K+ ATPase Pump restores the Na+ and K+ gradients within the neuron during the relative refractory period and enables the neuron to fully reset and fire another action potential.
The refractory period in one location in the neuron, also allows the directional movement of the depolarization event from the dendrites of the neuron and through the cell body. If the signal is strong enough to depolarize the neuron to a state of -55 mV when it reaches the axon hillock, this will generate an action potential that will be sent down the axon of the neuron. This is a committed step by the neuron that will cause the release of neurotransmitters into the synaptic cleft, signaling the activation of the downstream neuron or target cell.
Note that these are only two examples of how ion channels can be regulated. Some ion channels can also be activated by the physical movement of the cell, such as the hair cells located in the inner ear, or by other chemical changes such as phosphorylation. Some ion channels are leaky and are open all the time, allowing the slow, continual movement of ions across the membrane. This keeps the osmotic pressure under control and doesn’t allow it to build up to dangerous levels, similar to having the overflow valve on your bathtub, so that the water doesn’t get too high and overflow the bathtub.
Video Tutorial on the Action Potential
(Back to the Top)
4.11 Chapter Summary
If an atom has gained one or more electrons, it is negatively charged and is called an anion. If an atom has lost one or more electrons, it is positively charged and is called a cation. Metals generally form cations while nonmetals generally form anions. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds. The simplest ionic compounds are binary ionic compounds or those that only contain two atoms, one acting as the cation, and one acting as the anion.
The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the “Octet Rule.” The term isoelectronic refers to an atom and an ion of a different atom (or two different ions) that have the same electron configuration. Cations lose electrons to become isoelectronic with the noble gas in the previous row (period) on the table. Anions gain electrons to become isoelectronic with the noble gas in the same row as the anion. The periodic table can be used to predict common ion states for the elements
During ionic bond formation, electron dot diagrams can be used to illustrate electron movements. Stable ionic compounds have a balanced charge state such that the charge on the overall molecule is zero. When writing chemical formulas, the cation is always first and the anion is always last. Stable chemical formulas must be written so that the overall compound has a net neutral charge (ie the total positive charge = the total negative charge). Subscripts are used to show how many atoms are present within an ionic formula. Chemical formulas are always reduced to show the lowest number of each cation and anion required for a single compound to form.
Cations are named by using the element name followed by the word ‘ion’. Roman numerals are added after the element name if a cation has more than one ionic form. Anions are named by dropping the last part of the element name and replacing it with the suffix ‘-ide’ followed by the word ‘ion’. When naming an ionic compound the cation name, including roman numerals when needed, is placed first, followed by the anion name. Polyatomic ions are ions that form from multiple atoms that are covalently bonded together. Polyatomic ions behave as a single group when participating in ionic bonding. Naming ionic compounds that contain polyatomic ions is done in exactly the same way as with other binary ionic compounds. The name of the cation comes first (using roman numerals when necessary) followed by the name of the anion.
Solid ionic compounds typically form a continuous three-dimensional network or lattice, usually in a crystalline structure, rather than individual molecules. Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids, they are most often electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized. Mobilized ions in solution are called electrolytes.
Using the Arrhenius definitions, ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing hydroxide (OH−) or oxide (O2−) ions are classified as bases. All other ionic compounds without these ions are known as salts. Naming salts and basic ionic compounds follows standard ionic nomenclature rules. In naming acids from binary compounds, the prefix ‘hydro-‘ is used to represent the cation H+, and the suffix ‘-ic’ acid is used to indicate that it is an acidic form. If an acid contains a polyatomic ion, no leading prefix is used to indicate the H+ cation. This is implied within the name. For polyatomic anions ending with the suffix ‘-ate’, the acid is named as the [anion name] + the ‘-ic acid’ suffix. If a polyatomic anion has the ‘-ite’ ending, the acid name will be written as the [anion name] + the ‘-ous acid’ suffix. The prefixes ‘hypo-‘ and ‘per-‘ are also retained in the acid nomenclature for elements that have many oxyanion states.
Neurons are electrically excitable cells that use ion gradients to generate nerve impulses called action potentials. Ion gradients are set up within the neuron through the use of ion pumps such as the Na+/K+ ATPase Protein. Ion pumps use energy to transport ions across the cell membrane against their concentration gradient. This sets up a resting membrane potential within the neuron of -70 mV and a condition where Na+ is in high concentration outside of the cell and K+ is in high concentration inside of the cell. Ion channel proteins use facilitated diffusion to transport ions across the plasma membrane down their concentration gradient. Receptor/Ligand ion channels in the dendrites of the neuron bind with neurotransmitters and cause the depolarization of the localized region. Voltage-gated sodium channels are activated allowing Na+ to flow into the cell and cause further depolarization. If the cell is depolarized to -55 mV at the axon hillock, and action potential will be generated down the axon and neurotransmitters will be released by the neuron. The resting potential of the neuron is reset by the opening of voltage-gated potassium channels. The Na+/K+ ATPase pump is then utilized to reset the ion gradients to prepare the neuron for another signaling event. The whole process takes approximately 4-5 milliseconds. Overall, neural signaling in humans uses approximately 20% of total energy consumption.
(Back to the Top)
4.12 References
- Ball, D. W.; Hill J. W.; Scott, R. J. The Basics of General, Organic, and Biological Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry_Textbook_Maps/Map%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)
- Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
- Wikipedia. Mercury (element). Published under Creative Commons by-sa 3.0 Unported License. Available at: https://en.wikipedia.org/wiki/Mercury_(element)
- Wikipedia. St. Elmo’s fire. Published under Creative Commons by-sa 3.0 Unported License. Available at: https://en.wikipedia.org/wiki/St._Elmo’s_fire
- Bernhoft R. A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health. 2012, 2012, 1-10. Available from: https://www.hindawi.com/journals/jeph/2012/460508/abs/
- Wikipedia. Bromoperoxidase. Published under Creative Commons by-sa 3.0 Unported License. Available at: https://en.wikipedia.org/wiki/Bromoperoxidase
- Bewick, S., Parsons, R., Forsythe, T., Robinson, S., and Dupon, J. (2016) Introductory Chemistry (CK-12) LibreTexts. https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry_Textbook_Maps/Map%3A_Introductory_Chemistry_(CK-12)/08%3A_Ionic_and_Metallic_Bonding/8.03%3A_Cation_Formation
- General Chemistry/Naming Substances. (2017, March 16). Wikibooks, The Free Textbook Project. Retrieved 15:35, April 13, 2017 from https://en.wikibooks.org/w/index.php?title=General_Chemistry/Naming_Substances&oldid=3196789.
- Mercury(I) chloride. (2017, January 18). In Wikipedia, The Free Encyclopedia. Retrieved 18:21, April 14, 2017, from https://en.wikipedia.org/w/index.php?title=Mercury(I)_chloride&oldid=760689931
- Blue mass. (2016, October 2). In Wikipedia, The Free Encyclopedia. Retrieved 18:11, April 15, 2017, from https://en.wikipedia.org/w/index.php?title=Blue_mass&oldid=742175689
- Wikipedia contributors. (2018, December 13). Neuron. In Wikipedia, The Free Encyclopedia. Retrieved 22:45, December 13, 2018, from https://en.wikipedia.org/w/index.php?title=Neuron&oldid=873450803
- Openstax. (2016) Chapter 12: The Nervous System and Nervous Tissue. In Rice University’s Online Textbook, Anatomy and Physiology. Retrieved Dec 13th, 2018 from: https://opentextbc.ca/anatomyandphysiology/chapter/12-2-nervous-tissue/
- File:Sodium-Potassium Pump.jpg. (2017, January 3). Wikimedia Commons, the free media repository. Retrieved 00:26, December 14, 2018 from https://commons.wikimedia.org/w/index.php?title=File:Sodium-Potassium_Pump.jpg&oldid=228564972.
- Wikipedia contributors. (2018, December 8). Synapse. In Wikipedia, The Free Encyclopedia. Retrieved 03:15, December 14, 2018, from https://en.wikipedia.org/w/index.php?title=Synapse&oldid=872616292
(Back to the Top)