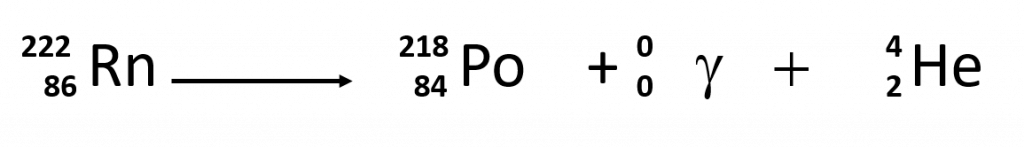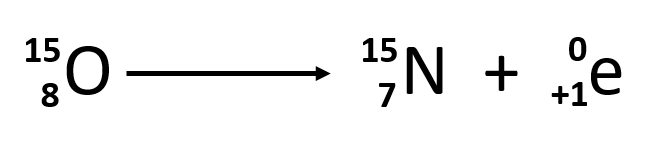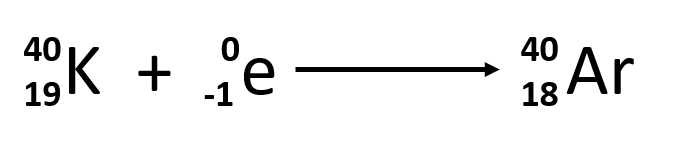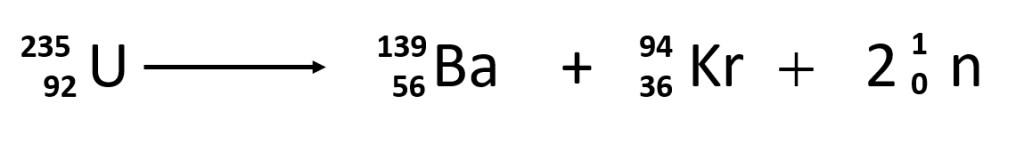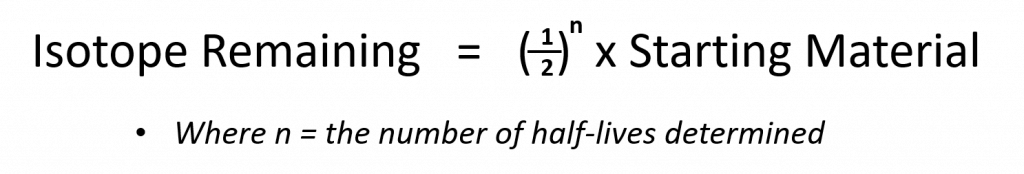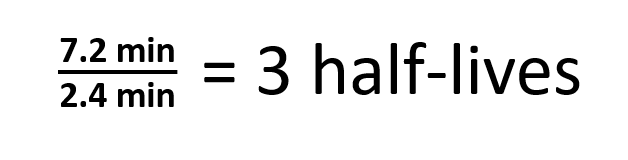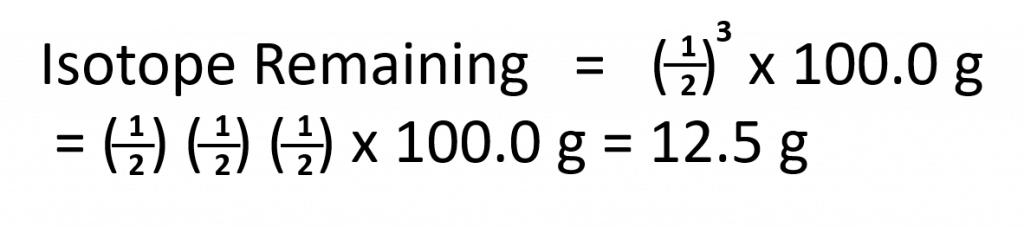Home » Student Resources » Online Chemistry Textbooks » CH103: Allied Health Chemistry » CH103 – CHAPTER 3: Radioactivity and Nuclear Chemistry
MenuCH103: Allied Health Chemistry
Radioactivity and Nuclear Chemistry
3.1 Major Forms of Radioactivity
Alpha Particle (α)
Beta Particle (β)
Gamma Radiation (γ)
Positron Emission (β+ decay) and Electron Capture
Nuclear Fission
3.2 Radioactive Half Lives
3.3 Biological Effects of Radiation Exposure
3.4 Uses of Radioactive Isotopes
3.5 Chapter Summary
3.6 References
Radioactivity and Nuclear Chemistry
Atomic theory in the nineteenth century presumed that nuclei had fixed compositions. But in 1896, the French scientist Henri Becquerel found that a uranium compound placed near a photographic plate made an image on the plate, even if the compound was wrapped in black cloth. He reasoned that the uranium compound was emitting some kind of radiation that passed through the cloth to expose the photographic plate. Further investigations showed that the radiation was a combination of particles and electromagnetic rays, with its ultimate source being the atomic nucleus. These emanations were ultimately called, collectively, radioactivity.
Following the somewhat serendipitous discovery of radioactivity by Becquerel, many prominent scientists began to investigate this new, intriguing phenomenon. Among them were Marie Curie (the first woman to win a Nobel Prize, and the only person to win two Nobel Prizes in different sciences—chemistry and physics), who was the first to coin the term “radioactivity,” and Ernest Rutherford (of gold foil experiment fame), who investigated and named three of the most common types of radiation. During the beginning of the twentieth century, many radioactive substances were discovered, the properties of radiation were investigated and quantified, and a solid understanding of radiation and nuclear decay was developed.
The spontaneous change of an unstable nuclide into another is radioactive decay. The unstable nuclide is called the parent nuclide; the nuclide that results from the decay is known as the daughter nuclide. The daughter nuclide may be stable, or it may decay itself. The radiation produced during radioactive decay is such that the daughter nuclide lies closer to the band of stability than the parent nuclide, so the location of a nuclide relative to the band of stability can serve as a guide to the kind of decay it will undergo (Figure 3.1).
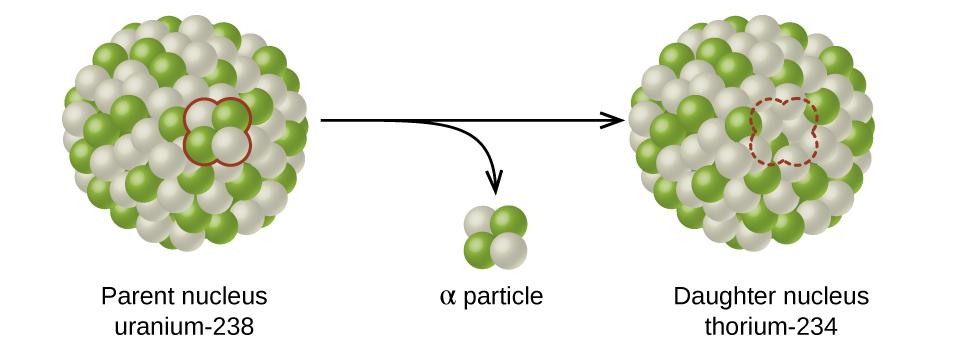
Figure 3.1 A nucleus of uranium-238 (the parent nuclide) undergoes α decay to form thorium-234 (the daughter nuclide). The alpha particle removes two protons (green) and two neutrons (gray) from the uranium-238 nucleus.
3.1 Major Forms of Radioactivity
Alpha Particle (α)
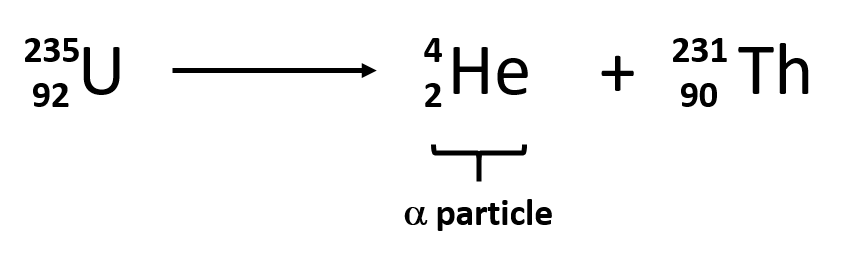
Rutherford’s experiments demonstrated that there are three main forms of radioactive emissions. The first is called an alpha particle, which is symbolized by the Greek letter α. An alpha particle is composed of two protons and two neutrons and is the same as a helium nucleus. (We often use 24He to represent an alpha particle.) It has a 2+ charge. When a radioactive atom emits an alpha particle, the original atom’s atomic number decreases by two (because of the loss of two protons), and its mass number decreases by four (because of the loss of four nuclear particles). We can represent the emission of an alpha particle with a chemical equation—for example, the alpha-particle emission of uranium-235 is as follows:
Rather than calling this equation a chemical equation, we call it a nuclear equation to emphasize that the change occurs in an atomic nucleus. How do we know that a product of this reaction is 90231Th? We use the law of conservation of matter, which says that matter cannot be created or destroyed. This means we must have the same number of protons and neutrons on both sides of the nuclear equation. If our uranium nucleus loses 2 protons, there are 90 protons remaining, identifying the element as thorium. Moreover, if we lose four nuclear particles of the original 235, there are 231 remaining. Thus we use subtraction to identify the isotope of the Th atom—in this case, 90231Th.
Beta Particle (β)
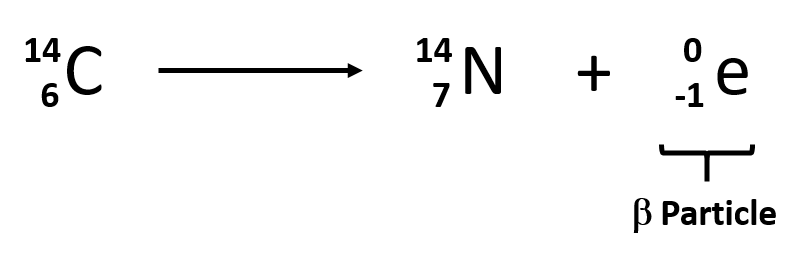
The second type of radioactive emission is called a beta particle, which is symbolized by the Greek letter β. A beta particle is an electron ejected from the nucleus (not from the shells of electrons about the nucleus) and has a -1 charge. We can also represent a beta particle as -10e. The net effect of beta particle emission on a nucleus is that a neutron is converted to a proton. The overall mass number stays the same, but because the number of protons increases by one, the atomic number goes up by one. Carbon-14 decays by emitting a beta particle:
Again, the sum of the atomic numbers is the same on both sides of the equation, as is the sum of the mass numbers. (Note that the electron is assigned an “atomic number” of –1, equal to its charge.)
Gamma Radiation (γ)
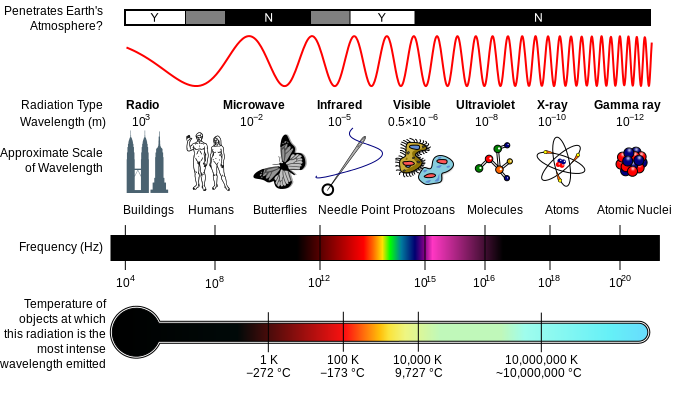
The third major type of radioactive emission is not a particle but rather a very energetic form of electromagnetic radiation called gamma rays, symbolized by the Greek letter γ. Electromagnetic radiation can be characterized into different categories based on the wavelength and photon energies. The electromagnetic spectrum shown in figure 3.2 shows the major categories of electromagnetic radiation. Note that the human sensory adaptations of sight and hearing have evolved to detect electromagnetic radiation, with radio waves having wavelengths between 1 mm and 100 km and visible light having wavelengths between 380 – 700 nm. Technological advances have helped humankind utilize other forms of electromagnetic radiation including X-rays and microwaves.
Figure 3.2 The Electromagnetic Spectrum. A diagram of the electromagnetic spectrum, showing various properties across the range of frequencies and wavelengths. Image Available from Wikipedia
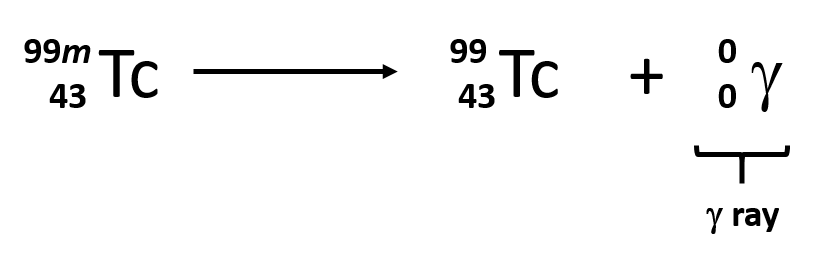
Some electromagnetic radiation with very short wavelengths are active enough that they may knock out electrons out of atoms in a sample of matter and make it electrically charged. The types of radiation that can do this are termed ionizing radiation. X-rays and Gamma rays are examples of ionizing radiation. Some radioactive materials, emit gamma radiation during their decay. For example, in the decay of radioactive technetium-99, a gamma ray is emitted. Note that in radioactive decay where the emission of gamma radiation occurs, that the identity of the parent material does not change, as no particles are physically emitted.
Sometimes the radioactive decay of a sample can result in the release of multiple forms of radioactivity. For example, in the radioactive decay of radon-222, both alpha and gamma radiation are emitted, with the latter having an energy of 8.2 × 10−14 J per nucleus decayed:
This may not seem like much energy, but if 1 mol of Rn atoms were to decay, the gamma ray energy would be 4.9 × 107 kJ!
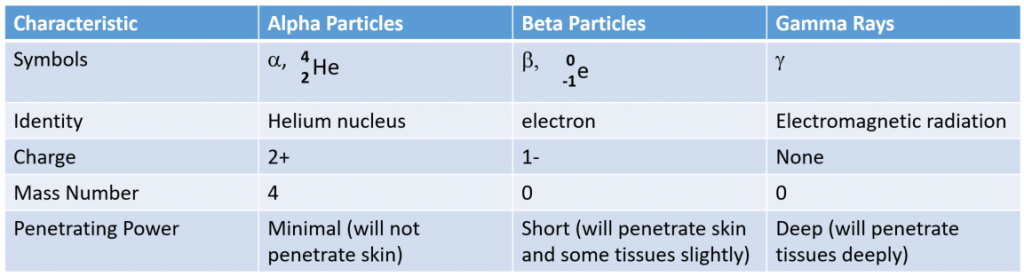
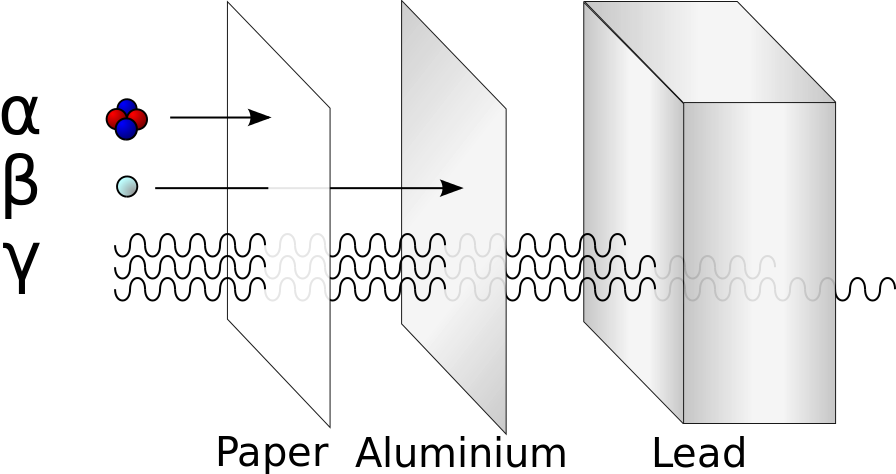
Alpha, beta, and gamma emissions have different abilities to penetrate matter. The relatively large alpha particle is easily stopped by matter (although it may impart a significant amount of energy to the matter it contacts). Beta particles penetrate slightly into matter, perhaps a few centimeters at most. Gamma rays can penetrate deeply into matter and can impart a large amount of energy into the surrounding matter. Table 3.1 summarizes the properties of the three main types of radioactive emissions and Figure 3.3 summarizes the ability of each radioactive type to penetrate matter.
Table 3.1 The Three Main Forms of Radioactive Emissions
Figure 3.3 Illustration of the relative abilities of three different types of ionizing radiation to penetrate solid matter. Typical alpha particles (α) are stopped by a sheet of paper, while beta particles (β) are stopped by an aluminum plate. Gamma radiation (γ) is damped when it penetrates lead. Figure provided by Stannered
Positron Emission (β+ decay) and Electron Capture
In addition to the three major types of radioactive particles listed above, two additional less common types of emissions have been discovered. These include positron emission and electron capture.
Positron emission (β+ decay) is the emission of a positron from the nucleus. Oxygen-15 is an example of a nuclide that undergoes positron emission:
Positron emission is observed for nuclides in which the n:p ratio is low. These nuclides lie below the band of stability. Positron decay is the conversion of a proton into a neutron with the emission of a positron. The n:p ratio increases, and the daughter nuclide lies closer to the band of stability than did the parent nuclide. The positron has the mass of an electron, but a positive charge. Thus, the overall mass of the nuclide doesn’t change, but the atomic number is decreased by one, which causes a change in the elemental identity of the daughter isotope.
Electron capture occurs when one of the inner electrons in an atom is captured by the atom’s nucleus. For example, potassium-40 undergoes electron capture:
Electron capture occurs when an inner shell electron combines with a proton and is converted into a neutron. The loss of an inner shell electron leaves a vacancy that will be filled by one of the outer electrons. As the outer electron drops into the vacancy, it will emit energy. In most cases, the energy emitted will be in the form of an X-ray. Like positron emission, electron capture occurs for “proton-rich” nuclei that lie below the band of stability. Electron capture has the same effect on the nucleus as does positron emission: The atomic number is decreased by one and the mass number does not change. This increases the n:p ratio, and the daughter nuclide lies closer to the band of stability than did the parent nuclide. Whether electron capture or positron emission occurs is difficult to predict. The choice is primarily due to kinetic factors, with the one requiring the smaller activation energy being the one more likely to occur.
Figure 3.4 summarizes these types of decay, along with their equations and changes in atomic and mass numbers.
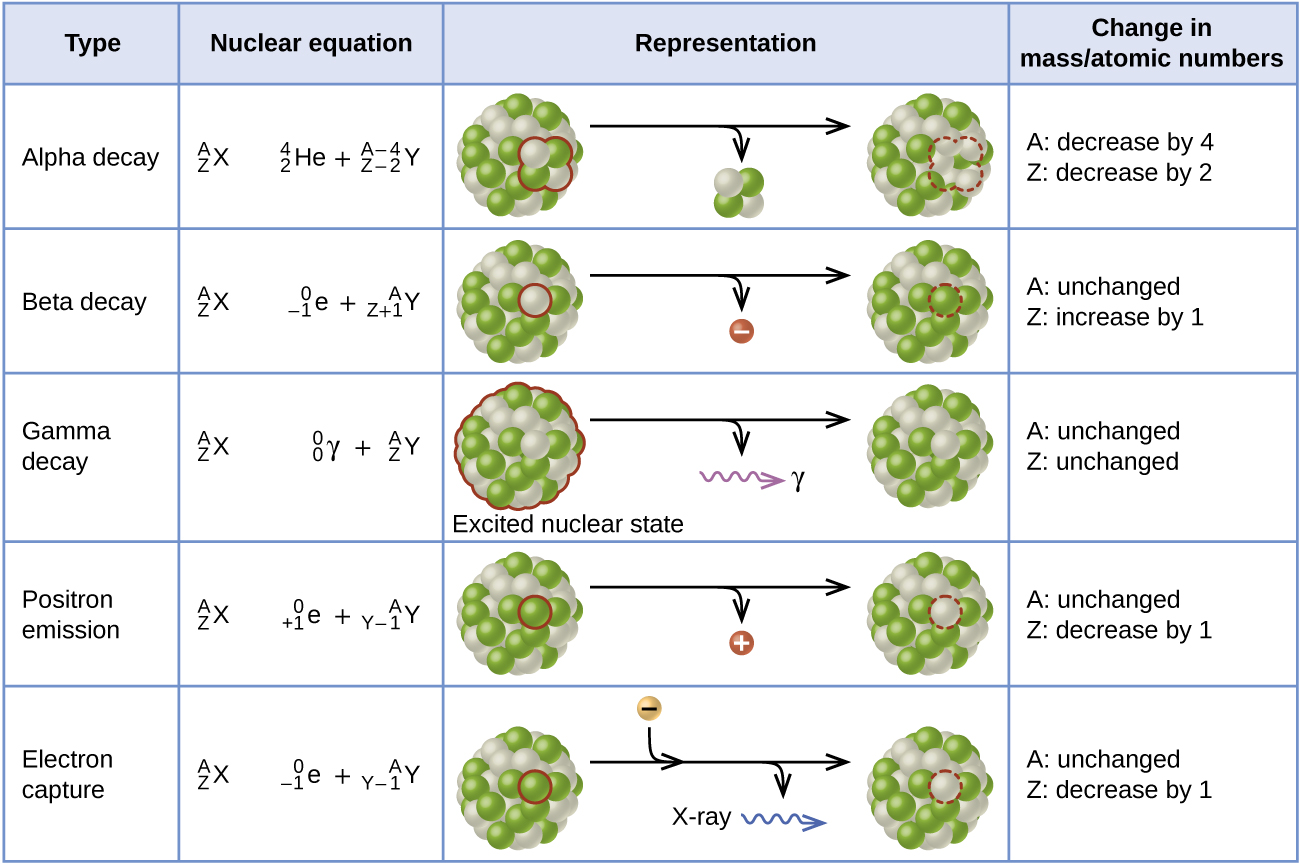
Figure 3.4. Summary of the type, nuclear equation, representation, and any changes in the mass or atomic numbers for various types of decay.
Nuclear Fission
Occasionally, an atomic nucleus breaks apart into smaller pieces in a radioactive process called spontaneous fission (or fission). Typically, the daughter isotopes produced by fission are a varied mix of products, rather than a specific isotope as with alpha and beta particle emission. Often, fission produces excess neutrons that will sometimes be captured by other nuclei, possibly inducing additional radioactive events. Uranium-235 undergoes spontaneous fission to a small extent. One typical reaction is
where 01n is a neutron. As with any nuclear process, the sums of the atomic numbers and mass numbers must be the same on both sides of the equation. Spontaneous fission is found only in large nuclei. The smallest nucleus that exhibits spontaneous fission is lead-208. (Fission is the radioactive process used in nuclear power plants and one type of nuclear bomb.)
(Back to the Top)
3.2 Radioactive Half Lives
Each radioactive nuclide has a characteristic, constant half-life (t1/2), the time required for half of the atoms in a sample to decay. An isotope’s half-life allows us to determine how long a sample of a useful isotope will be available, and how long a sample of an undesirable or dangerous isotope must be stored before it decays to a low-enough radiation level that is no longer a problem.
For example, cobalt-60, an isotope that emits gamma rays used to treat cancer, has a half-life of 5.27 years (Figure 3.5). In a given cobalt-60 source, since half of the nuclei decay every 5.27 years, both the amount of material and the intensity of the radiation emitted is cut in half every 5.27 years. Note that for a given substance, the intensity of radiation that it produces is directly proportional to the rate of decay of the substance and the amount of the substance. Thus, a cobalt-60 source that is used for cancer treatment must be replaced regularly to continue to be effective.
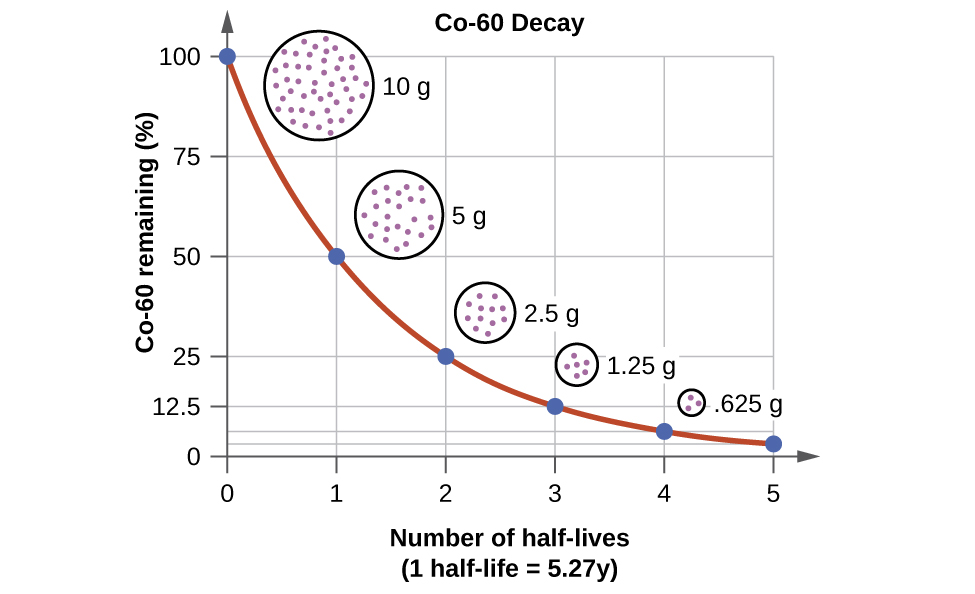
Figure 3.5. The Decay of Cobalt-60. For cobalt-60, which has a half-life of 5.27 years, 50% remains after 5.27 years (one half-life), 25% remains after 10.54 years (two half-lives), 12.5% remains after 15.81 years (three half-lives), and so on. Note that every half-life is the same length of time.
Since every half-life for a radionuclide is the same length of time, we can use the following equation to calculate how much radioactive nuclide is remaining after the passage of any number (n) of half-lives:
Practice Problem:
Question: The half-life of Zn-71 is 2.4 minutes. If one had 100.0 g at the beginning, how many grams would be left after 7.2 minutes has elapsed?
Solution:
Step 1. Determine the number of half-lives that have passed: number of half-lives = time passed divided by the half-life (Be sure that the time units match!!)
Step 2. Use the Isotope Remaining equation to solve for how much isotope will remain after the number of half-lives determined in step 1 have passed.
(Back to the Top)
3.3 Biological Effects of Radiation Exposure
There is a large difference in the magnitude of the biological effects of nonionizing radiation (for example, light and microwaves) and ionizing radiation, emissions energetic enough to knock electrons out of molecules (for example, α and β particles, γ rays, X-rays, and high-energy ultraviolet radiation) (Figure 3.6).
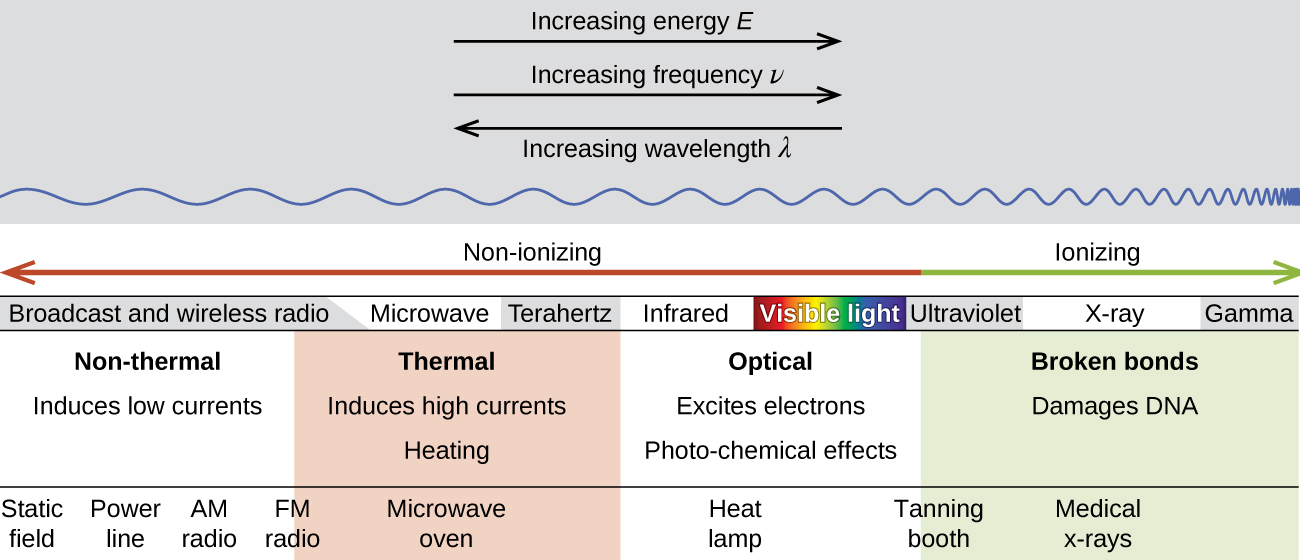
Figure 3.6. Damaging Effects of Ionizing Radiation. Lower frequency, lower-energy electromagnetic radiation is nonionizing, and higher frequency, higher-energy electromagnetic radiation is ionizing.
Energy absorbed from nonionizing radiation speeds up the movement of atoms and molecules, which is equivalent to heating the sample. Although biological systems are sensitive to heat (as we might know from touching a hot stove or spending a day at the beach in the sun), a large amount of nonionizing radiation is necessary before dangerous levels are reached. Ionizing radiation, however, may cause much more severe damage by breaking bonds or removing electrons in biological molecules, disrupting their structure and function (Figure 3.7).
Radiation can harm either the whole body (somatic damage) or eggs and sperm (genetic damage). Its effects are more pronounced in cells that reproduce rapidly, such as the stomach lining, hair follicles, bone marrow, and embryos. This is why patients undergoing radiation therapy often feel nauseous or sick to their stomach, lose hair, have bone aches, and so on, and why particular care must be taken when undergoing radiation therapy during pregnancy.
(Back to the Top)
3.4 Uses of Radioactive Isotopes
Radioactive isotopes have the same chemical properties as stable isotopes of the same element, but they emit radiation, which can be detected. If we replace one (or more) atom(s) with radioisotope(s) in a compound, we can track them by monitoring their radioactive emissions. This type of compound is called a radioactive tracer (or radioactive label). Radioisotopes are used to follow the paths of biochemical reactions or to determine how a substance is distributed within an organism. Radioactive tracers are also used in many medical applications, including both diagnosis and treatment. They are also used in many other industries to measure engine wear, analyze the geological formation around oil wells, and much more.
Radioisotopes have revolutionized medical practice, where they are used extensively. Over 10 million nuclear medicine procedures and more than 100 million nuclear medicine tests are performed annually in the United States. Four typical examples of radioactive tracers used in medicine are technetium-99 , thallium-201
, iodine-131
, and sodium-24
. Damaged tissues in the heart, liver, and lungs absorb certain compounds of technetium-99 preferentially. After it is injected, the location of the technetium compound, and hence the damaged tissue, can be determined by detecting the γ rays emitted by the Tc-99 isotope. Thallium-201 (Figure 3.8) becomes concentrated in healthy heart tissue, so the two isotopes, Tc-99 and Tl-201, are used together to study heart tissue. Iodine-131 concentrates in the thyroid gland, the liver, and some parts of the brain. It can therefore be used to monitor goiter and treat thyroid conditions, such as Grave’s disease, as well as liver and brain tumors. Salt solutions containing compounds of sodium-24 are injected into the bloodstream to help locate obstructions to the flow of blood.
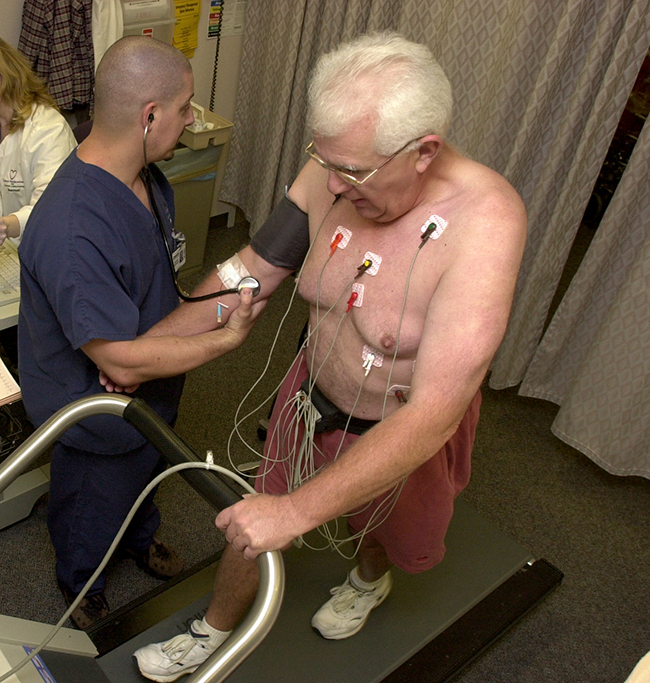
Figure 3.8. Administering thallium-201 to a patient and subsequently performing a stress test offer medical professionals an opportunity to visually analyze heart function and blood flow. (credit: modification of work by “Blue0ctane”/Wikimedia Commons)
Radioisotopes used in medicine typically have short half-lives—for example, Tc-99 has a half-life of 6.01 hours. This makes Tc-99 essentially impossible to store and prohibitively expensive to transport, so it is made on-site instead. Hospitals and other medical facilities use Mo-99 (which is primarily extracted from U-235 fission products) to generate Tc-99. Mo-99 undergoes β decay with a half-life of 66 hours, and the Tc-99 is then chemically extracted (Figure 3.9). The parent nuclide Mo-99 is part of a molybdate ion, ; when it decays, it forms the pertechnetate ion,
. These two water-soluble ions are separated by column chromatography, with the higher charge molybdate ion adsorbing onto the alumina in the column, and the lower charge pertechnetate ion passing through the column in the solution. A few micrograms of Mo-99 can produce enough Tc-99 to perform as many as 10,000 tests.
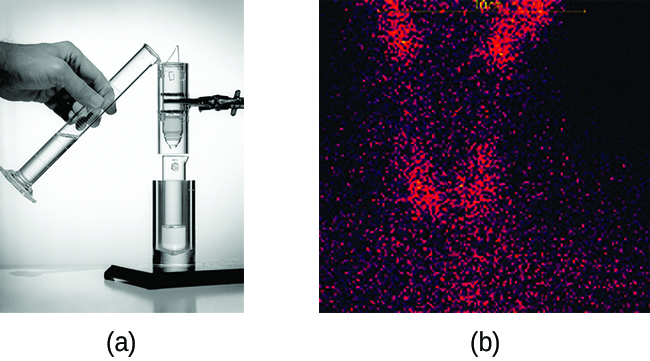
Figure 3.9. (a) The first Tc-99m generator (circa 1958) is used to separate Tc-99 from Mo-99. The MoO42- is retained by the matrix in the column, whereas the TcO4–. passes through and is collected. (b) Tc-99 was used in this scan of the neck of a patient with Grave’s disease. The scan shows the location of high concentrations of Tc-99. (credit a: modification of work by the Department of Energy; credit b: modification of work by “MBq”/Wikimedia Commons)
Positron emission tomography (PET) scans use radiation to diagnose and track health conditions and monitor medical treatments by revealing how parts of a patient’s body function (Figure 3.10). To perform a PET scan, a positron-emitting radioisotope is produced in a cyclotron and then attached to a substance that is used by the part of the body being investigated. This “tagged” compound, or radiotracer, is then administered to the patient (injected via IV or breathed in as a gas), and how it is used by the tissue reveals how that organ or other area of the body functions.
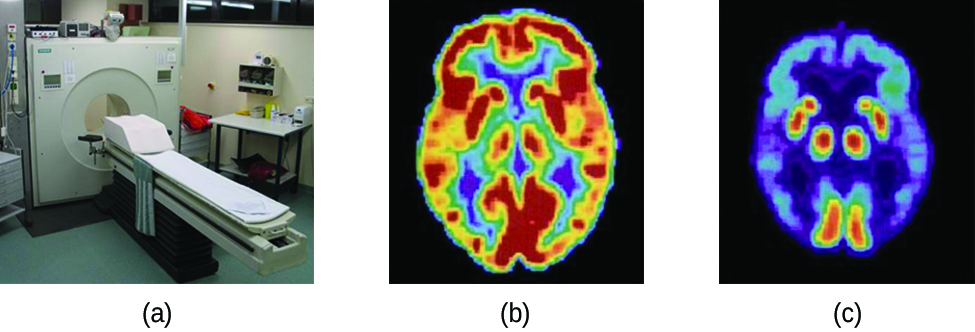
Figure 3.10. A PET scanner (a) uses radiation to provide an image of how part of a patient’s body functions. The scans it produces can be used to image a healthy brain (b) or can be used for diagnosing medical conditions such as Alzheimer’s disease (c). (credit a: modification of work by Jens Maus)
For example, F-18 is produced by proton bombardment of 18O () and incorporated into a glucose analog called fludeoxyglucose (FDG). How FDG is used by the body provides critical diagnostic information; for example, since cancers use glucose differently than normal tissues, FDG can reveal cancers. The 18F emits positrons that interact with nearby electrons, producing a burst of gamma radiation. This energy is detected by the scanner and converted into a detailed, three-dimensional, color image that shows how that part of the patient’s body functions. Different levels of gamma radiation produce different amounts of brightness and colors in the image, which can then be interpreted by a radiologist to reveal what is going on. PET scans can detect heart damage and heart disease, help diagnose Alzheimer’s disease, indicate the part of a brain that is affected by epilepsy, reveal cancer, show what stage it is, and how much it has spread, and whether treatments are effective. Unlike magnetic resonance imaging and X-rays, which only show how something looks, the big advantage of PET scans is that they show how something functions. PET scans are now usually performed in conjunction with a computed tomography scan.
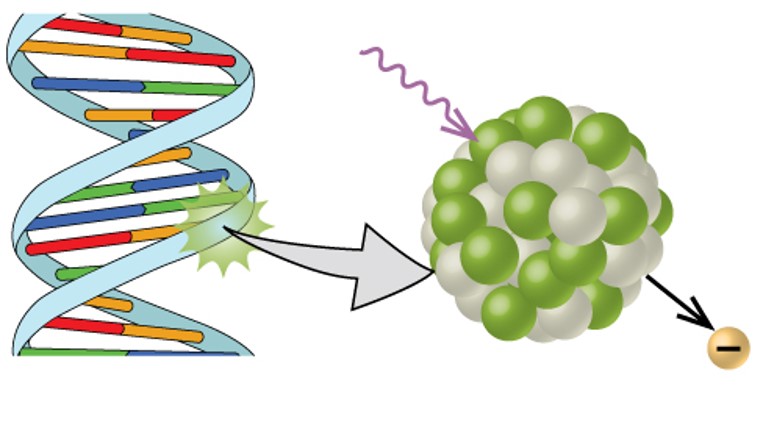
Radioisotopes can also be used, typically in higher doses than as a tracer, as treatment. Radiation therapy is the use of high-energy radiation to damage the DNA of cancer cells, which kills them or keeps them from dividing (Figure 3.11). A cancer patient may receive external beam radiation therapy delivered by a machine outside the body, or internal radiation therapy (brachytherapy) from a radioactive substance that has been introduced into the body. Note that chemotherapy is similar to internal radiation therapy in that the cancer treatment is injected into the body, but differs in that chemotherapy uses chemical rather than radioactive substances to kill the cancer cells.
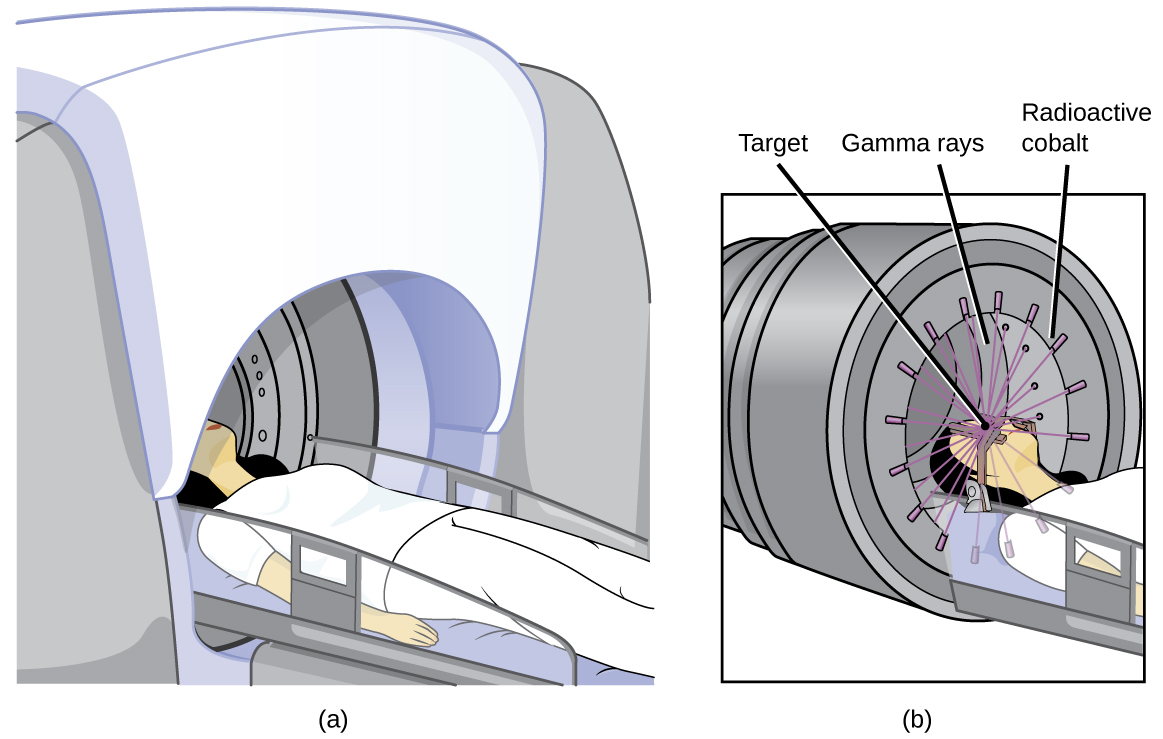
Figure 3.11. The cartoon in (a) shows a cobalt-60 machine used in the treatment of cancer. The diagram in (b) shows how the gantry of the Co-60 machine swings through an arc, focusing radiation on the targeted region (tumor) and minimizing the amount of radiation that passes through nearby regions.
Cobalt-60 is a synthetic radioisotope produced by the neutron activation of Co-59, which then undergoes β decay to form Ni-60, along with the emission of γ radiation. The overall process is:
The overall decay scheme for this is shown graphically in Figure 3.12.
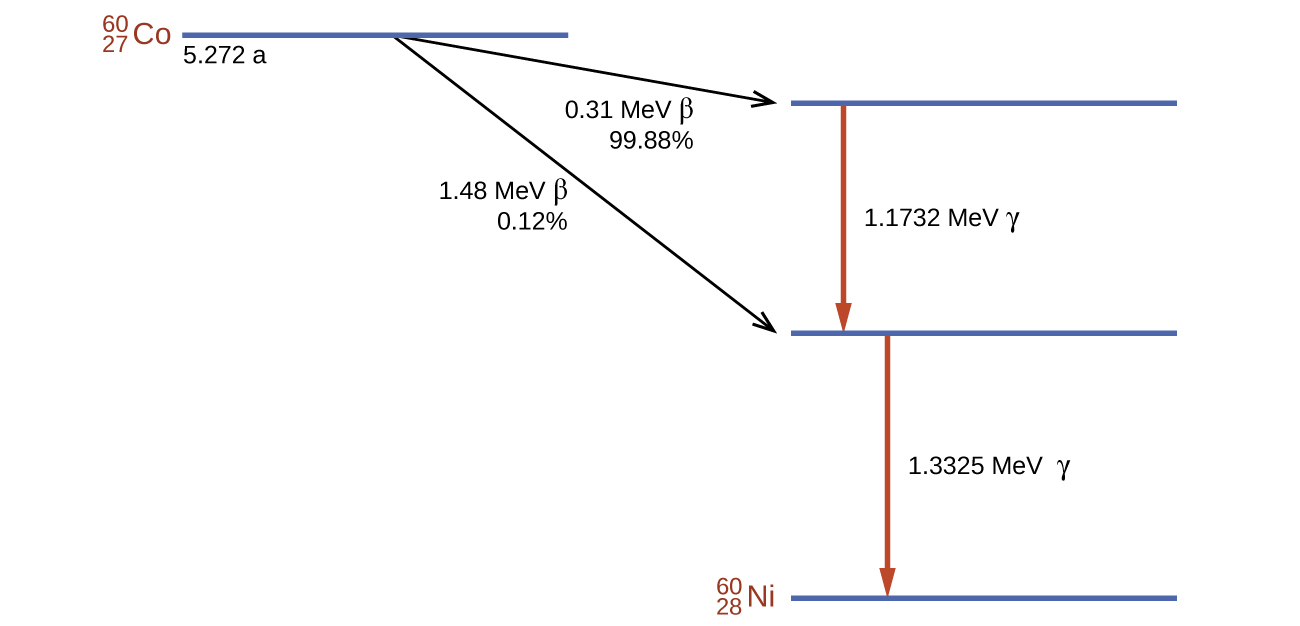
Figure 3.12. Co-60 undergoes a series of radioactive decays. The γ emissions are used for radiation therapy.
Radioisotopes are used in diverse ways to study the mechanisms of chemical reactions in plants and animals. These include labeling fertilizers in studies of nutrient uptake by plants and crop growth, investigations of digestive and milk-producing processes in cows, and studies on the growth and metabolism of animals and plants.
For example, the radioisotope C-14 was used to elucidate the details of how photosynthesis occurs. The overall reaction is:
but the process is much more complex, proceeding through a series of steps in which various organic compounds are produced. In studies of the pathway of this reaction, plants were exposed to CO2 containing a high concentration of . At regular intervals, the plants were analyzed to determine which organic compounds contained carbon-14 and how much of each compound was present. From the time sequence in which the compounds appeared and the amount of each present at given time intervals, scientists learned more about the pathway of the reaction.
Commercial applications of radioactive materials are equally diverse (Figure 3.13). They include determining the thickness of films and thin metal sheets by exploiting the penetration power of various types of radiation. Flaws in metals used for structural purposes can be detected using high-energy gamma rays from cobalt-60 in a fashion similar to the way X-rays are used to examine the human body. In one form of pest control, flies are controlled by sterilizing male flies with γ radiation so that females breeding with them do not produce offspring. Many foods are preserved by radiation that kills microorganisms that cause the foods to spoil.

Figure 3.13. Common commercial uses of radiation include (a) X-ray examination of luggage at an airport and (b) preservation of food. (credit a: modification of work by the Department of the Navy; credit b: modification of work by the US Department of Agriculture)
Americium-241, an α emitter with a half-life of 458 years, is used in tiny amounts in ionization-type smoke detectors (Figure 3.14). The α emissions from Am-241 ionize the air between two electrode plates in the ionizing chamber. A battery supplies a potential that causes movement of the ions, thus creating a small electric current. When smoke enters the chamber, the movement of the ions is impeded, reducing the conductivity of the air. This causes a marked drop in the current, triggering an alarm.
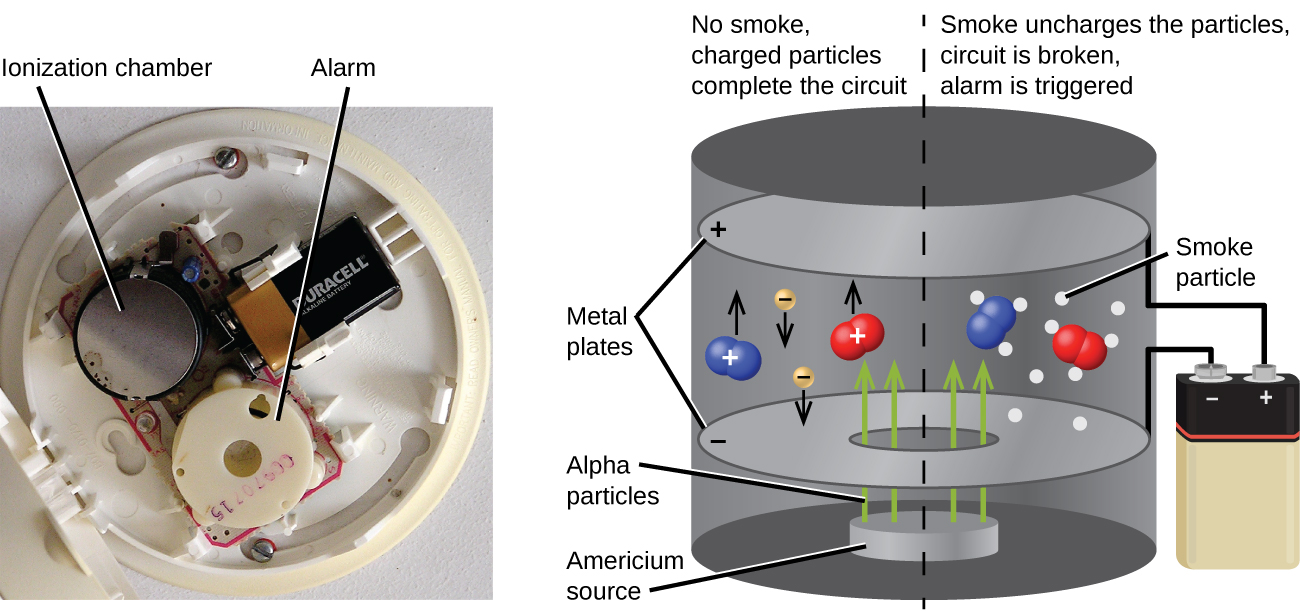
Figure 3.14. Inside a smoke detector, Am-241 emits α particles that ionize the air, creating a small electric current. During a fire, smoke particles impede the flow of ions, reducing the current and triggering an alarm. (credit a: modification of work by “Muffet”/Wikimedia Commons)
(Back to the Top)
3.5 Chapter Summary
Radioactivity is defined as the emission of particles and electromagnetic rays from the nucleus of an unstable atom. Six types of radiation produced during nuclear decay were presented within this chapter and include:
- alpha (α) decay which is composed of two protons and two neutrons and has a +2 charge.
- beta (β) decay which is an electron ejected from the nucleus (not from the shells of electrons about the nucleus) and has a -1 charge and no mass. Within the nucleus a neutron emits the electron and is converted into a proton in the process.
- gamma (γ) decay which is characterized by the emission of ionizing radiation and does not contain mass or charge.
- positron (β+) emission which is a positron ejected from the nucleus and has a +1 charge and no mass. Within the nucleus a proton emits the positron and is converted into a neutron in the process.
- electron capture occurs when an inner shell electron combines with a proton and is converted into a neutron. The loss of an inner shell electron leaves a vacancy that will be filled by one of the outer electrons. As the outer electron drops into the vacancy, it will emit energy often in the form of X-rays.
- nuclear fission occurs when an atomic nucleus breaks apart into smaller pieces in a radioactive process that releases excess neutrons.
Each radioactive nuclide has a characteristic, constant half-life (t1/2), the time required for half of the atoms in a sample to decay. The equation below can be used to determine how much isotope will remain after the passage of a given number of half-lives
Radioactive emissions can cause damage to biological systems by causing the breakdown of proteins and DNA. This can lead to cellular and genetic damage and increase a person’s risk for diseases like cancer. However, when used is small quantities and in controlled settings, radioactive tracers and treatments have proven to be revolutionary for the medical field. For example, Radiation therapy is the use of high-energy radiation to damage the DNA of cancer cells, which kills them or keeps them from dividing. Radioactive tracers have also been very useful in evaluating heart disease, thyroid dysfunction, and other blood disorders. Positron emission tomography (PET) scans use radiation to diagnose and track health conditions and monitor medical treatments by revealing how parts of a patient’s body function and X-rays have long been used to visualize breaks in bones and cavities in teeth.
(Back to the Top)
3.6 References
Unless otherwise noted, resources for this chapter have been modified from the following creative commons resources:
- OpenStax . (2016) Chapter 21 – Nuclear Chemistry. Chemistry by Rice University is licensed under a Creative Commons Attribution 4.0 International Accessed, Dec 1st, 2018 from: https://opentextbc.ca/chemistry/chapter/introduction-2/