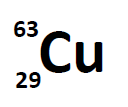Home » Student Resources » Online Chemistry Textbooks » CH103: Allied Health Chemistry » CH103 – CHAPTER 2: Atoms and the Periodic Table
MenuCH103: Allied Health Chemistry
Chapter 2: Atoms and the Periodic Table
Section 2.1: Chemistry and Matter
What is Chemistry?
Physical and Chemical Properties
Elements and Compounds
Mixtures
States of Matter
Reactions in Chemistry
Section 2.2: How Scientists Study Chemistry
The Scientific Method
2.3 Atomic Theory with Historical Perspectives
2.4 Introduction to Elements and the Periodic Table
2.5 Dmitri Mendeleev and the development of the periodic table
2.6 Families of the Periodic Table
2.7 Defining the Atom
Basic Atomic Structure – electrons, neutrons, and protons
2.8 Atomic Number – Protons Determine the Identity of an Element
2.9 Atomic Mass, Isotopes, and Molar Mass
2.10 Periodic Table Trends
Atomic Size
Electronegativity
Ionization Energy
Metallic and Nonmetallic Character
2.11 Chapter Summary and Homework
2.12 References
Section 2.1: Chemistry and Matter
What is Chemistry?
Everything around us is made up of chemicals. From the color that makes a rose so red to the gasoline that fills our cars and the silicon chips that power our computers and cell phones…Chemistry is everywhere! Understanding how chemical molecules form and interact to create complex structures enables us to harness the power of chemistry and use it, just like a toolbox, to create many of the modern advances that we see today. This includes advances in medicine, communication, transportation, building infrastructure, food science and agriculture, and nearly every other technical field that you can imagine.
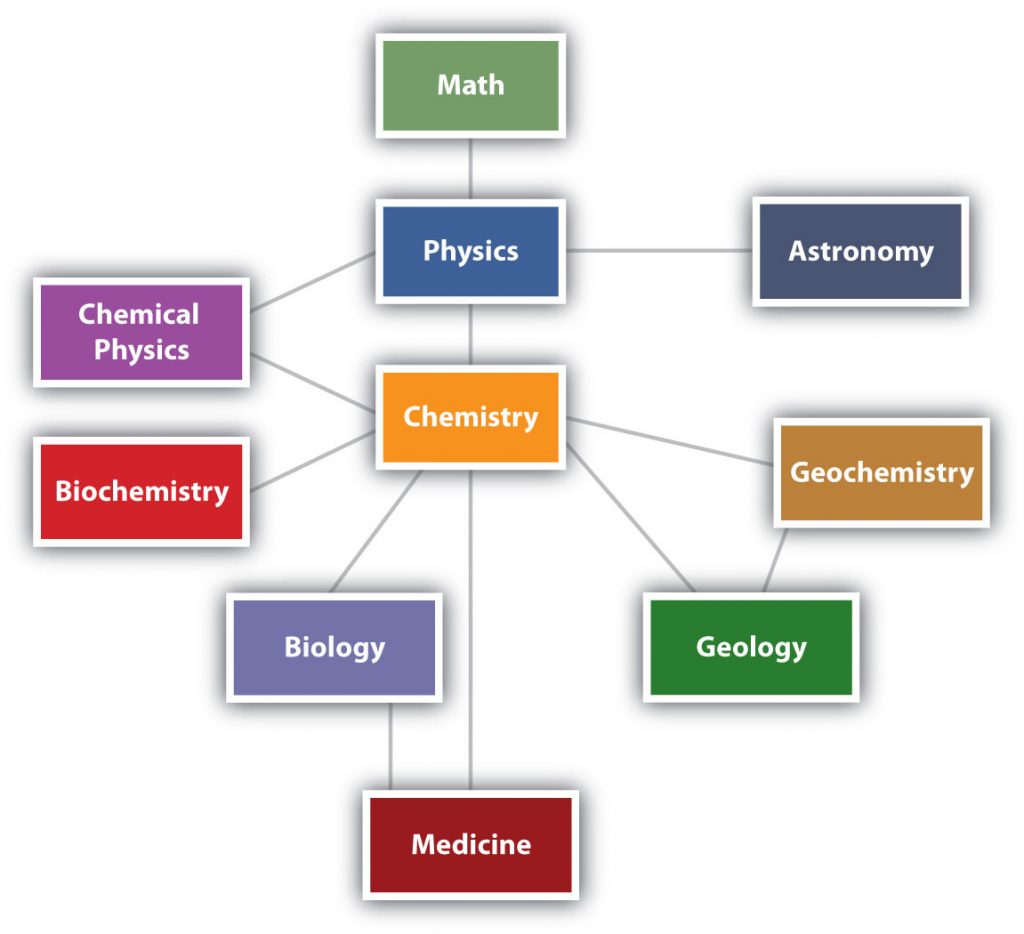
Chemistry is one branch of science. Science is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. Because the physical universe is so vast, there are many different branches of science (Figure 2.1). Thus, chemistry is the study of matter, biology is the study of living things, and geology is the study of rocks and the earth. Mathematics and Physics are the languages of science, and we will use them to communicate some of the ideas of chemistry.
Although we divide science into different fields, there is much overlap among them. For example, some biologists and chemists work in both fields so much that their work is called biochemistry. Similarly, geology and chemistry overlap in the field called geochemistry. Figure 2.1 shows how many of the individual fields of science are related.
Figure 2.1: The Relationships Between Some of the Major Branches of Science. Chemistry lies more or less in the middle, which emphasizes its importance to many branches of science.
Physical vs. Chemical Properties
Part of understanding matter is being able to describe it. One way chemists describe matter is to assign different kinds of properties to different categories. The properties that chemists use to describe matter fall into two general categories. Physical properties are characteristics that describes matter, such as boiling point, melting point and color. Physical Changes, such as melting a solid into a liquid, do not alter the chemical structure of that matter. Chemical properties are characteristics that describe how the chemical structure of matter changes during a chemical reaction. An example of a chemical property is flammability—a materials ability to burn—because burning (also known as combustion) changes the chemical composition of a material.
Elements and Compounds
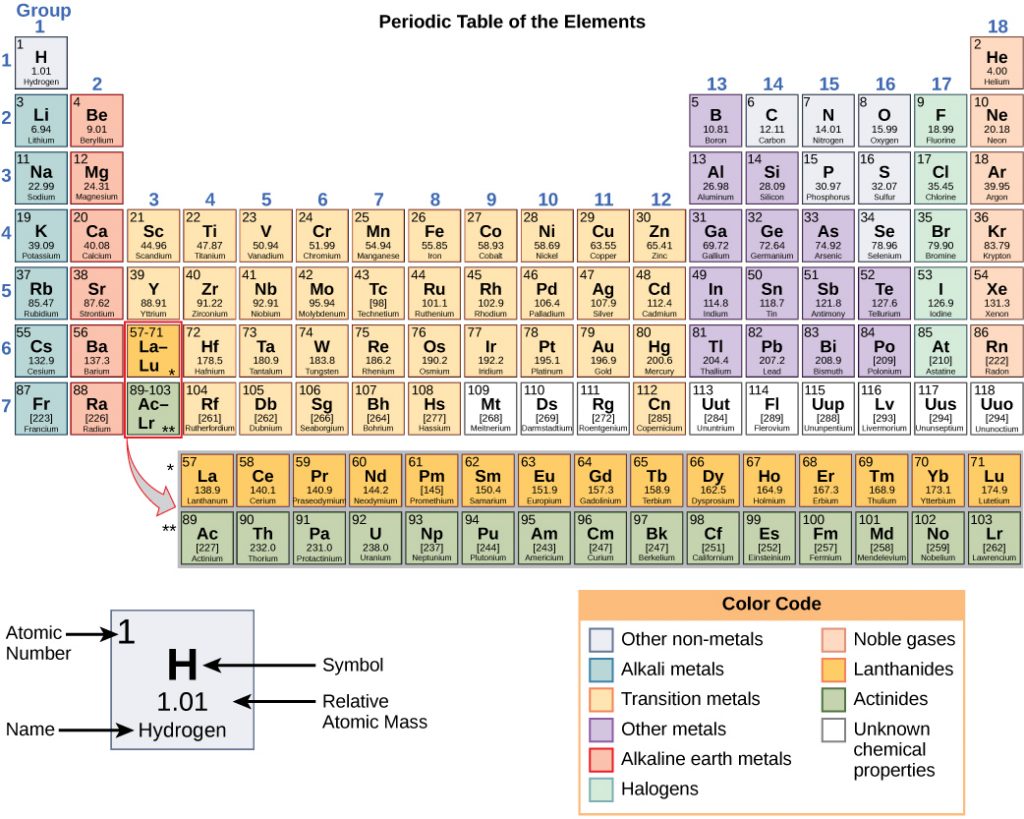
Any sample of matter that has the same physical and chemical properties throughout the sample is called a substance. There are two types of substances. A substance that cannot be broken down into chemically simpler components is an element. Aluminum, which is used in soda cans, is an element. A substance that can be broken down into chemically simpler components (because it has more than one element) is a compound. Water is a compound composed of the elements hydrogen and oxygen. Today, there are about 118 elements in the known universe which are organized on a fundamental chart called the Periodic Table of Elements (Fig. 2.2). In contrast, scientists have identified tens of millions of different compounds to date.
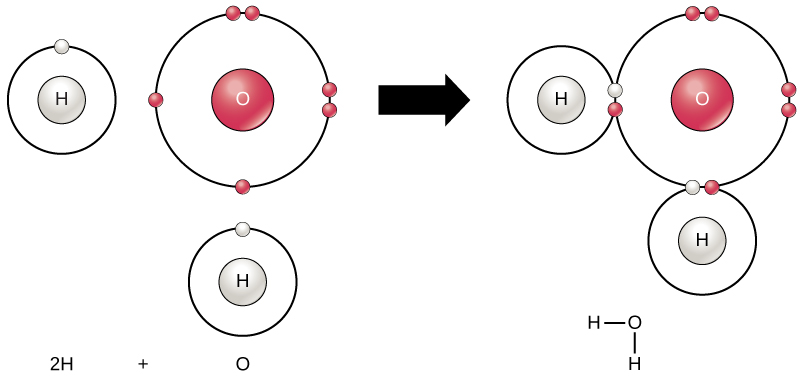
The smallest part of an element that maintains the identity of that element is called an atom. Atoms are extremely tiny; to make a line 1 inch long, you would need 217 million iron atoms! Similarly, the smallest part of a compound that maintains the identity of that compound is called a molecule. Molecules are composed of atoms that are attached together and behave as a unit (Fig. 2.2). Scientists usually work with millions of atoms and molecules at a time. When a scientist is working
Figure 2.2: (Upper Panel) The Periodic Table of the Elements is an organized chart that contains all of the known chemical elements. (Lower Panel) To the left of the arrow is shown one atom of oxygen and two atoms of hydrogen. Each of these represent single elements. When they are combined on the righthand side, they form a single molecule of water (H2O). Note that water is defined as a compound, because each single molecule is made up of more than one type of element, in this case, one atom of oxygen with two atoms of hydrogen.
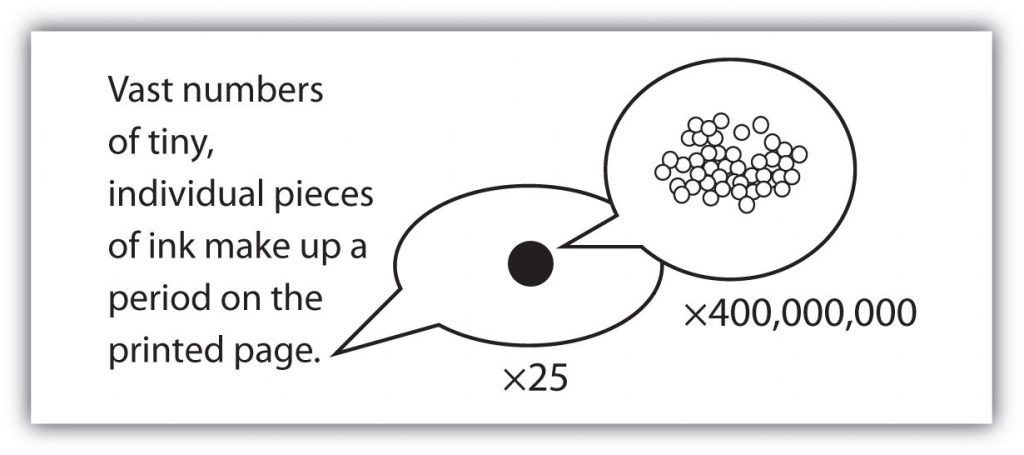
with large numbers of atoms or molecules at a time, the scientist is studying the macroscopic view of the universe. However, scientists can also describe chemical events on the level of individual atoms or molecules, which is referred to as the microscopic viewpoint. We will see examples of both macroscopic and microscopic viewpoints throughout this book (Figure 2.3).
Figure 2.3: How many molecules are needed for a period in a sentence? Although we do not notice it from a macroscopic perspective, matter is composed of microscopic particles so tiny that billions of them are needed to make a speck that we can see with the naked eye. The X25 and X400,000,000 indicate the number of times the image is magnified.
Mixtures
A material composed of two or more substances is a mixture. In a mixture, the individual substances maintain their chemical identities. Many mixtures are obvious combinations of two or more substances, such as a mixture of sand and water. Such mixtures are called heterogeneous mixtures. In some mixtures, the components are so intimately combined that they act like a single substance even though they are not. Mixtures with a consistent composition throughout are called homogeneous mixtures Homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other are called solutions. Sugar dissolved in water is an example of a solution. A metal alloy, such as steel, is an example of a solid solution. Air, a mixture of mainly nitrogen and oxygen, is a gaseous solution.
Figure 2.4: Heterogeneous vs. Homogeneous Mixtures. A mixture contains more than one substance. In the upper panel you see an example of a heterogeneous mixture of oil and water. The mixture is heterogeneous because you can visibly see two different components in the mixture. In the lower panel, you see an example of a homogeneous mixture, coffee. It is homogeneous because you cannot distinguish the many different components that make up a cup of coffee (water; caffeine; coffee alkaloids and tannins). It looks the same throughout. If the mixture is homogeneous and is also see through or clear, it is called a solution. In our example, the coffee is a solution; however, a concentrated espresso may be very opaque and would only be homogeneous mixture, not a solution.
States of Matter
Another way to classify matter is to describe it as a solid, a liquid, or a gas, which was done in the examples of solutions, above. These three descriptions, each implying that the matter has certain physical properties, represent the three phases of matter. A solid has a definite shape and a definite volume. Liquids have a definite volume but not a definite shape; they take the shape of their containers. Gases have neither a definite shape nor a definite volume, and they expand to fill their containers. We encounter matter in each phase every day. In fact, we regularly encounter water in all three phases: ice (solid), water (liquid), and steam (gas).
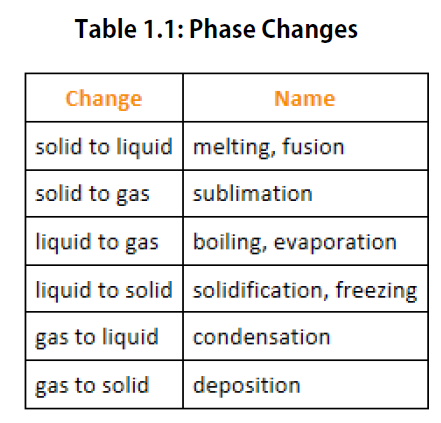
We know from our experience with water that substances can change from one phase to another if the conditions are right. Typically, varying the temperature of a substance (and, less commonly, the pressure exerted on it) can cause a phase change or a physical process in which a substance goes from one phase to another (Figure 2.5). Phase changes have particular names depending on what phases are involved, as summarized in Table 1.1.
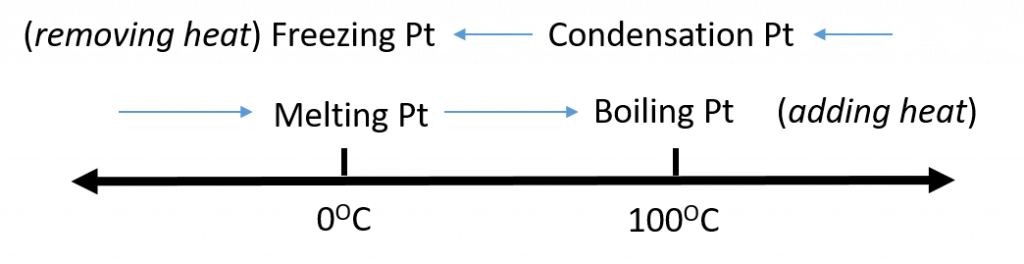
Figure 2.5. Analyzing Phase Changes. (Upper panel) A photo of boiling water demonstrates the phase change of water from the liquid to the gaseous phase. Note that phase changes are a physical property of a molecule. The water is still chemically the same (H2O) in the solid, liquid, or gaseous state. (Lower panel) Change in temperature can cause phase changes . Above is the temperature scale for the phase changes of water. If you add heat to solid ice, water will melt at 0oC and boil at 100oC. If you remove heat from gaseous water, it will condense into the liquid state at 100oC and freeze at 0oC.
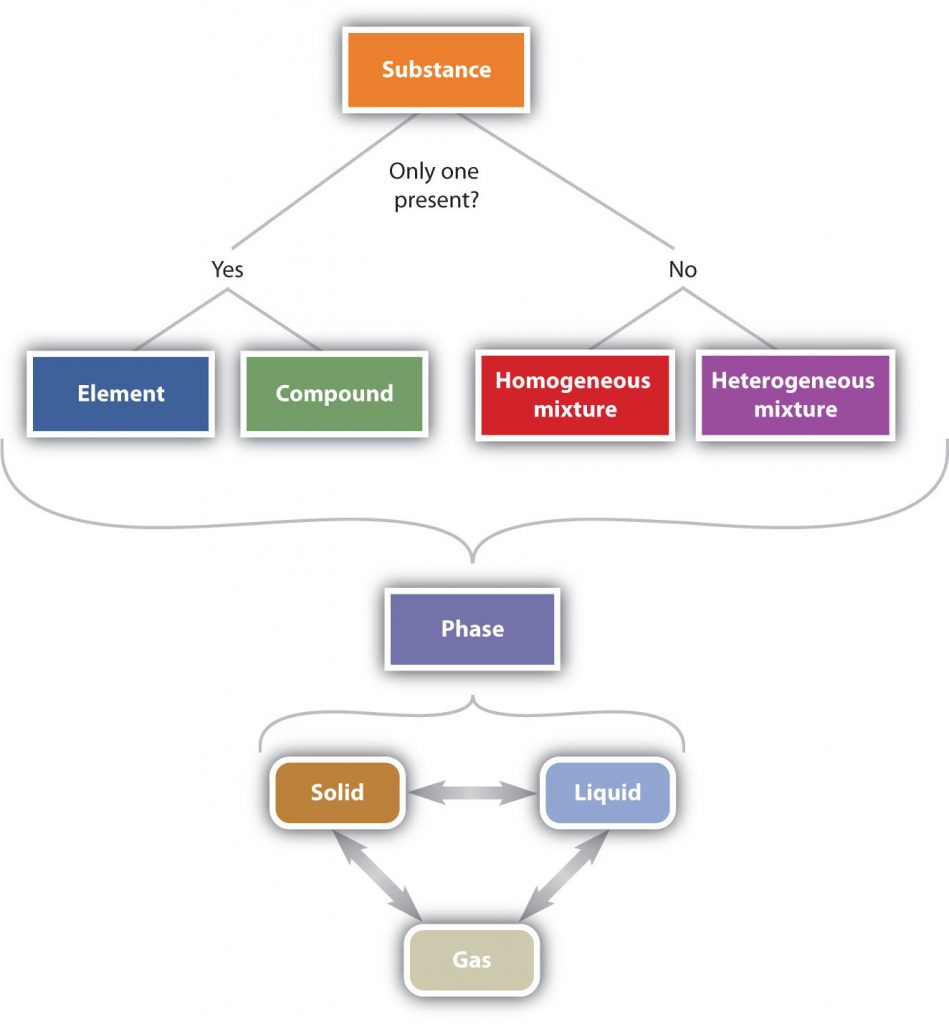
In summary, Figure 2.6 “The Classification of Matter” illustrates the relationships between the different ways matter can be classified.
Figure 2.6 The Classification of Matter. Matter can be classified in a variety of ways depending on its properties
Reactions in Chemistry
Atoms can react chemically with one another to form new compounds and arrangements. When chemical reactions are written, they share some conventional features: (1) The reactants are written on the left hand side of the equation, (2) The products are written on the right hand side of the equation, (3) The reactants and products are separated by an arrow that shows the direction of the reaction, note that some reactions are reversible and are denoted by a double-headed arrow, (4) Conditions that are required for the reaction, such as heat or a catalyst (an agent that speeds up the chemical reaction without being used up in the process) are typically indicated above the arrow of the reaction, and (5) the reactants and products can be fully written out, or they can be represented by their chemical structures or by their chemical abbreviations (which you will learn how to formulate in the following chapters).
Reactions are written out with the quantities of the reactants and products in mind as well. During a chemical reaction, matter can never be destroyed. Thus, the atoms that are present on the left hand side of the equation must also be on the right hand side of the equation. They can be rearranged or in different forms, but they must be present. This is known as the law of conservation of matter. To conform to this law, reactions are always written with the minimal amount of reactants required to make the minimal amount of products. An example of a chemical reaction is given below in Figure 2.7
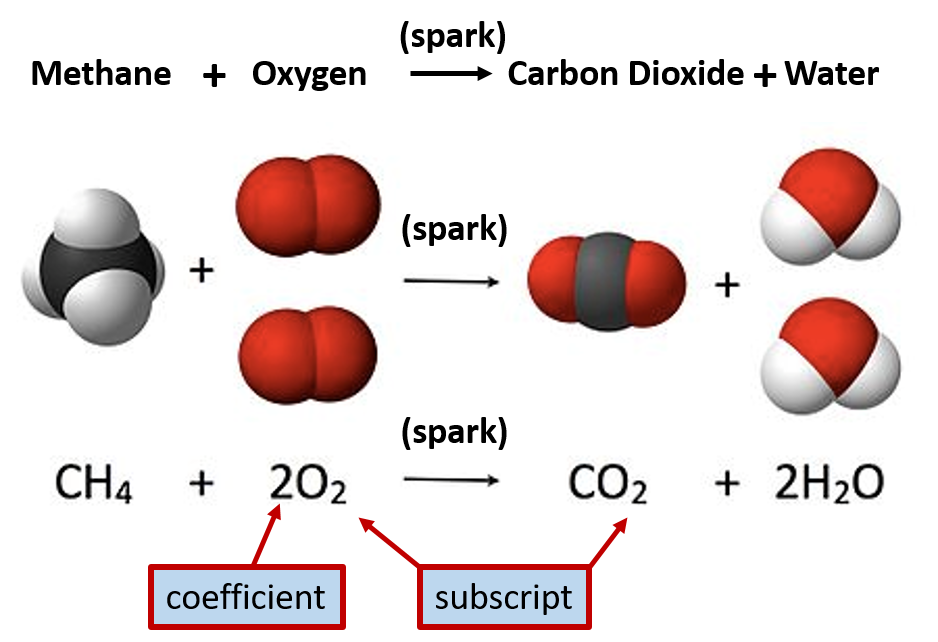
Figure 2.7 Representations of Chemical Reactions. In the example above the compound methane reacts with oxygen to form the products, carbon dioxide and water. Methane is also known as natural gas and is commonly burned to release usable energy. Subscripts present in the equation represent how many atoms of that element are involved with a chemical bond. If there is no number listed, the number one is implied. (ie in the carbon dioxide (CO2) molecule denoted above, there is one atom of carbon (C) bonded with two atoms of oxygen (O) to form one molecule of carbon dioxide.) Note that to begin the burning process, a spark is needed to get the reaction started. This spark is noted over the arrow, as it is not a reactant or a product of the reaction.
This figure is adapted from Jynto and Jynto (2013).
To represent the amounts of atoms present within a chemical reactions, numbers are present as either coefficients or subscripts within the equation. When the number is represented as a subscript, this indicates how many atoms of that element are required to make up a single molecule of the compound. In Figure 2.7, the chemical formula for carbon dioxide is given as CO2 The number 2 subscript next to the oxygen atom indicates that there are two atoms of oxygen within a single molecule of carbon dioxide. When there is no subscript present, this means that there is only one atom present. Thus, for one molecule of carbon dioxide, CO2, there is one atom of carbon and two atoms of oxygen. The coefficients in front of each substance, indicates how many molecules are required for a single reaction. In Figure 2.7, one molecule of methane reacts with 2 molecules of oxygen to yield one molecule of carbon dioxide and two molecules of water.
Since molecules are so small, it is impossible to ever measure out a single molecule when conducting an experiment in a laboratory. Thus, chemists will work with mole quantities of chemicals. The coefficients within chemical equations are also equivalent to mole quantities of each substance. Thus, the equation in Figure 2.7 can also be read as, one mole of methane reacts with 2 moles of oxygen to yield one mole of carbon dioxide and two moles of water.
Practice Problems for Section 2.1:
Section 2.2: How Scientists Study Chemistry
The Scientific Method
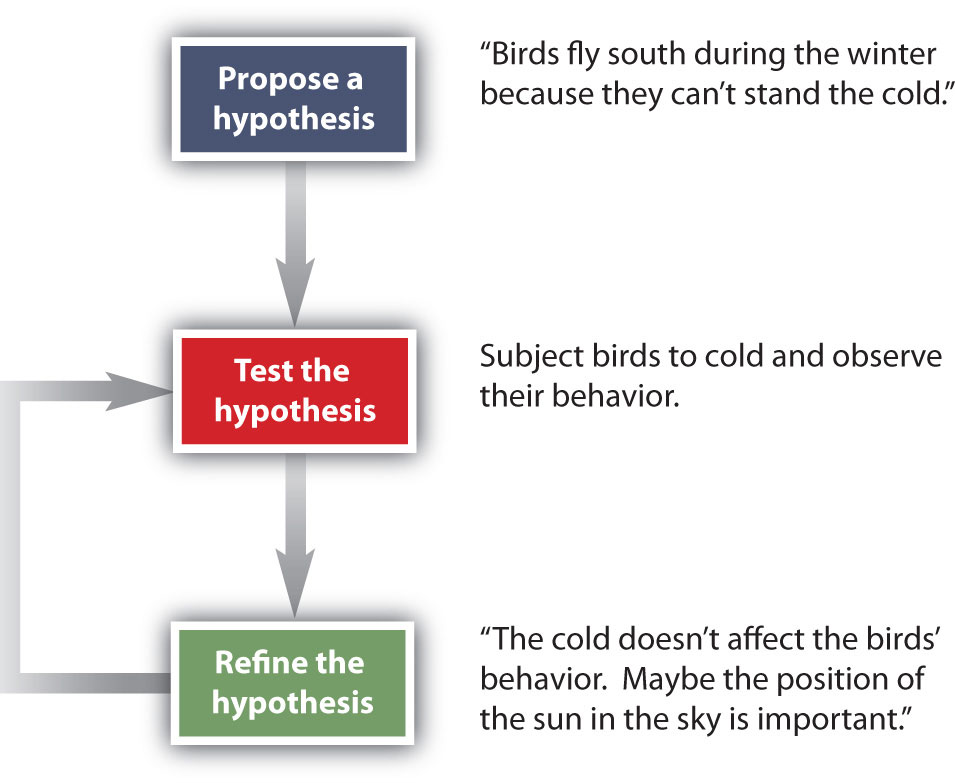
How do scientists work? Generally, they follow a process called the scientific method. The scientific method is an organized procedure for learning answers to questions. To find the answer to a question (for example, “Why do birds fly toward Earth’s equator during the cold months?”), a scientist goes through the following steps, which are also illustrated in Figure 2.8.
Figure 2.8 The General Steps of the Scientific Method. The steps may not be as clear-cut in real life as described here, but most scientific work follows this general outline.
Propose a hypothesis. A scientist generates a testable idea, or hypothesis, to try to answer a question or explain how the natural universe works. Some people use the word theory in place of hypothesis, but the word hypothesis is the proper word in science. For scientific applications, the word theory is a general statement that describes a large set of observations and data. A theory represents the highest level of scientific understanding, and is built from a wide array of factual knowledge or data.
Test the hypothesis. A scientist evaluates the hypothesis by devising and carrying out experiments to test it. If the hypothesis passes the test, it may be a proper answer to the question. If the hypothesis does not pass the test, it may not be a good answer.
Refine the hypothesis if necessary. Depending on the results of experiments, a scientist may want to modify the hypothesis and then test it again. Sometimes the results show the original hypothesis to be completely wrong, in which case a scientist will have to devise a new hypothesis.
Not all scientific investigations are simple enough to be separated into these three discrete steps. But these steps represent the general method by which scientists learn about our natural universe.
Practice Problems for Section 2.2
2.3 Atomic Theory with Historical Perspectives
What are the smallest building blocks of everyday objects? This is a question that has interested man since the age of the Greek philosophers. Like the ancient Greeks we can perform a simple thought experiment that raises a very important question for modern chemistry: suppose you were given a piece of aluminum foil and asked to cut the foil in half over and over. How long could you continue cutting, assuming that you had no limitations based on your own abilities? Is there a limit on how small matter can be broken up into, or could you infinitely divide matter into smaller and smaller pieces? This argument dates as far back as the Greek philosophers. Most, like Aristotle, argued that matter could be divided infinitely. However, one brilliant philosopher, Democritus, argued that there is a limit. He proposed that the smallest piece that any element (like aluminum) can be divided into and still be recognized as that element is an Atom, a word derived from the Greek word atomos, meaning “indivisible”.
Figure 2.9: Democritus
Photo taken from: Public Domain
Philosophers, like Democritus (Figure 2.9), based most of their ideas off of thought experiments like the one above instead of actual observations and experimentation. It is for this reason that Democritus’ ideas on atoms were dismissed until 1808, when John Dalton, an English scientist, proposed four fundamental assumptions based upon observations that we call Dalton’s Atomic Theory.
Dalton proposed that:
-
Matter is made up of tiny particles called atoms
-
Atoms cannot be broken into smaller pieces. During a chemical reaction, atoms are rearranged, but they do not break apart, nor are they created or destroyed
-
All atoms of the same element are identical in mass and other properties
-
Atoms of different elements differ in mass and other properties
2.4 Introduction to Elements and the Periodic Table
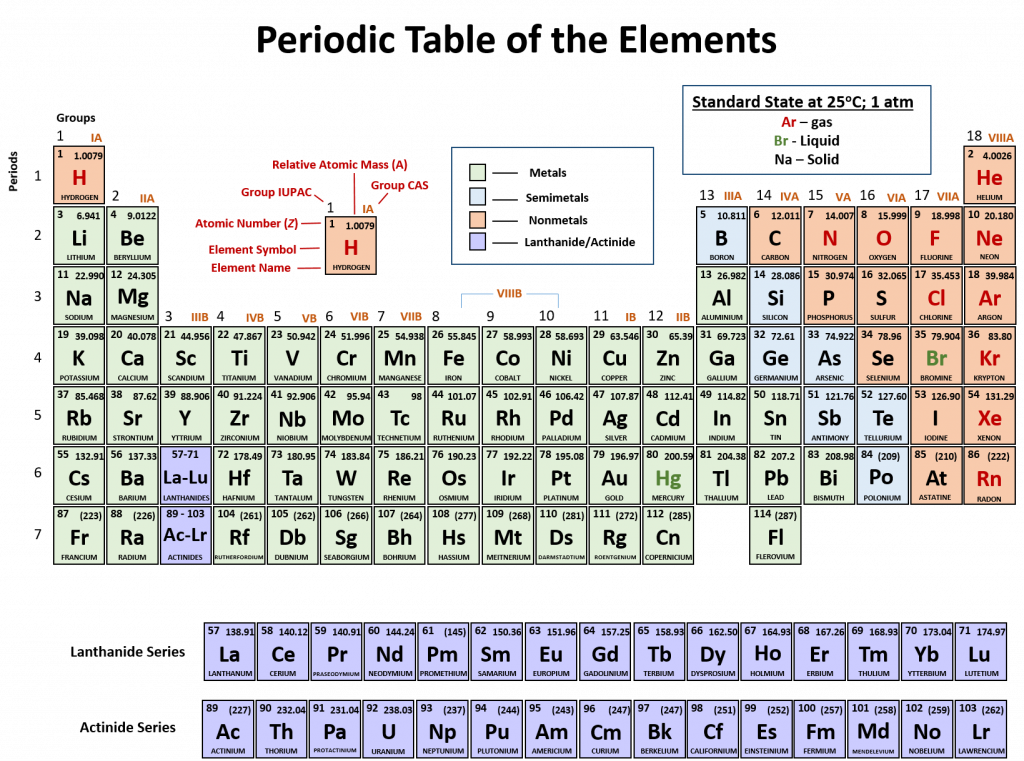
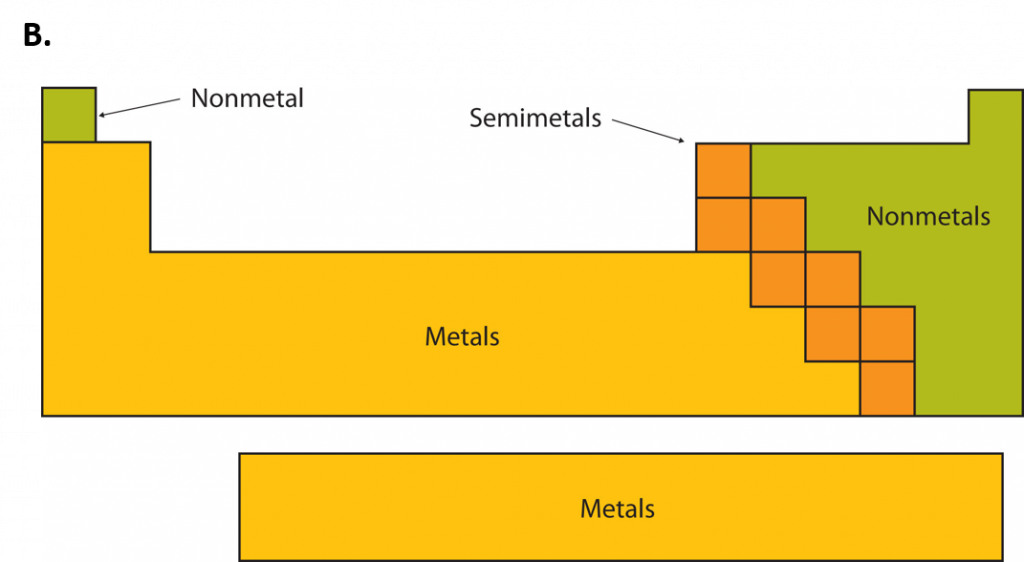
An element is a substance that cannot be broken down into simpler chemical substances. There are about 90 naturally occurring elements known on Earth. Using technology, scientists have been able to create nearly 30 additional elements that are not readily found in nature. Today, chemistry recognizes a total of 118 elements which are all represented on a standard chart of the elements, called the Periodic Table of Elements (Figure 2.9). Each element is represented by a one or two letter code, where the first letter is always capitalized and, if a second letter is present, it is written in lowercase. For example, the symbol for Hydrogen is H, and the symbol for carbon is C. Some of the elements have seemingly strange letter codes, such as sodium which is Na. These letter codes are derived from latin terminology. For example, the symbol for sodium (Na) is derived from the latin word, natrium, which means sodium carbonate. Elements in the periodic table can be broken up into different general classes based upon similarities in their properties. Going from left to right across the periodic table, the elements can be broken up into metals, metalloids, and nonmetals.
Metals are typically shiny, very dense, and have high melting points. Most metals are ductile (can be drawn out into thin wires), malleable (can be hammered into thin sheets), and good conductors of both heat as well as electricity. All metals are solids at room temperature except for mercury. In chemical reactions, metals easily lose electrons to form positive ions. Examples of metals are silver, gold, and zinc.
Nonmetals are generally brittle, dull, have low melting points, and they are generally poor conductors of heat as well as electricity. In chemical reactions, they tend to gain electrons to form negative ions. Examples of nonmetals are hydrogen, carbon, and nitrogen.
Metalloids have properties of both metals and nonmetals. Metalloids can be shiny or dull. Electricity and heat can travel through metalloids, although not as easily as they can through metals. They are also called semimetals. They are typically semi-conductors, which means that they are elements that conduct electricity better than insulators, but not as well as conductors. They are valuable in the computer chip industry. Examples of metalloids are silicon and boron.
A.
Periodic Table Downloadable PDF Version
Figure 2.10: Periodic Table of the Elements. All of the known chemical elements are arranged in the format of a table. The table has been set up in such a way that the characteristics of each different element can be predicted by their position on the table. (A) On this rendition of the periodic table, you can see that the pink elements on the lefthand side of the table are the metals, while the blue elements on the right are the non-metals (Hydrogen is the only exception to this rule and will be explained in the subsequent sections). The metalloids (also termed semi-metals) occur in a stairstep pattern between the metals and nonmetals and are represented in this diagram by the green elements. (B) Shows the positions of the metals, nonmetals and metalloids on the periodic table. During this chapter, you will learn more about these unique characteristics, called periodic trends.
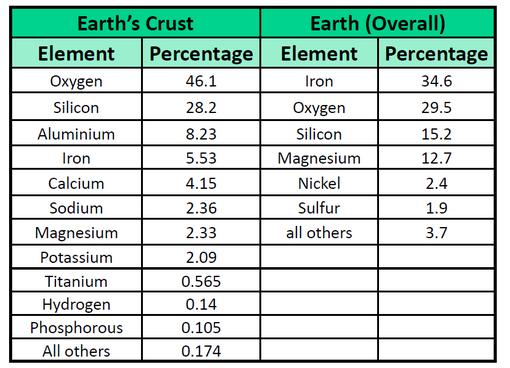
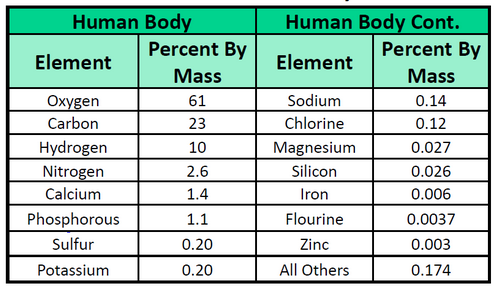
The elements vary widely in abundance. In the universe as a whole, the most common element is hydrogen (about 90%), followed by helium (most of the remaining 10%). All other elements are present in relatively minuscule amounts, as far as we can detect. On the planet Earth, however, the situation is rather different. Oxygen makes up 46.1% of the mass of Earth’s crust (the relatively thin layer of rock forming Earth’s surface), mostly in combination with other elements, while silicon makes up 28.5%. Hydrogen, the most abundant element in the universe, makes up only 0.14% of Earth’s crust. Table 1.2 lists the relative abundances of elements on Earth as a whole and in Earth’s crust. Table 1.3 lists the relative abundances of elements in the human body. If you compare Table 1.2 and 1.3, you will find disparities between the percentage of each element in the human body and on Earth. Oxygen has the highest percentage in both cases, but carbon, the element with the second highest percentage in the body, is relatively rare on Earth and does not even appear as a separate entry in Table 1.2; carbon is part of the 0.174% representing “other” elements. How does the human body concentrate so many apparently rare elements?
The relative amounts of elements in the body have less to do with their abundances on Earth than with their availability in a form we can assimilate. We obtain oxygen from the air we breathe and the water we drink. We also obtain hydrogen from water. On the other hand, although carbon is present in the atmosphere as carbon dioxide, and about 80% of the atmosphere is nitrogen, we obtain those two elements from the food we eat, not the air we breathe.
Table 1.2 Elemental Composition of the Earth
Table 1.3 Elemental Composition of the Human Body
Source of Tables 1.2 and 1.3: D.R. Lide, ed. CRC Handbook of Chemistry and Physics 89th ed. (Boca Raton, FL: CRC Press, 2008-9), 7-24.
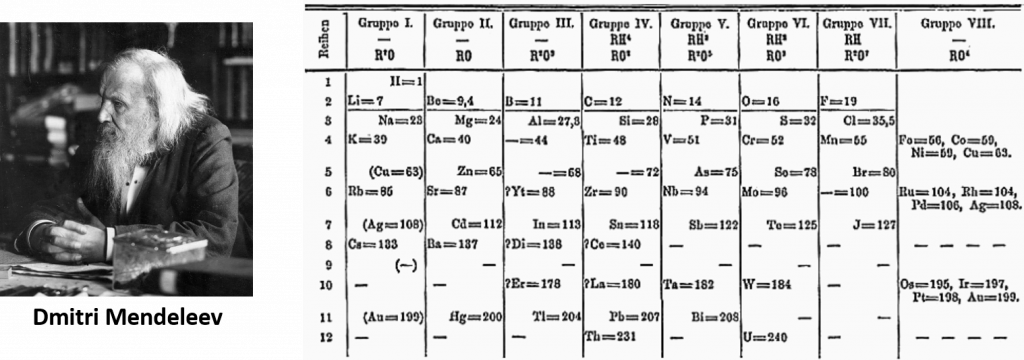
2.5 Dmitri Mendeleev and the development of the periodic table
In the 19th century, many previously unknown elements were discovered, and scientists noted that certain sets of elements had similar chemical properties. For example, chlorine, bromine, and iodine react with other elements (such as sodium) to make similar compounds. Likewise, lithium, sodium, and potassium react with other elements (such as oxygen) to make similar compounds. Why is this so?
In 1864, Julius Lothar Meyer, a German chemist, organized the elements by atomic mass and grouped them according to their chemical properties. Later that decade, Dmitri Mendeleev, a Russian chemist, organized all the known elements according to similar properties. He left gaps in his table for what he thought were undiscovered elements, and he made some bold predictions regarding the properties of those undiscovered elements. Later, when elements were discovered whose properties closely matched Mendeleev’s predictions, his version of the table gained favor in the scientific community. Because certain properties of the elements repeat on a regular basis throughout the table (that is, they are periodic), it became known as the periodic table (Figure 2.11).
Figure 2.11: Dmitri Medeleev’s 1871 Early Version of the Periodic Table
Photo of Dmitri Medeleev provided by: кабинет академика Михаила Михайловича Шульца – фото любезно передано мне в собственность вдовой М.М.Шульца Ниной Дмитриевной Шульц
Periodic Table provided by: Den fjättrade ankan
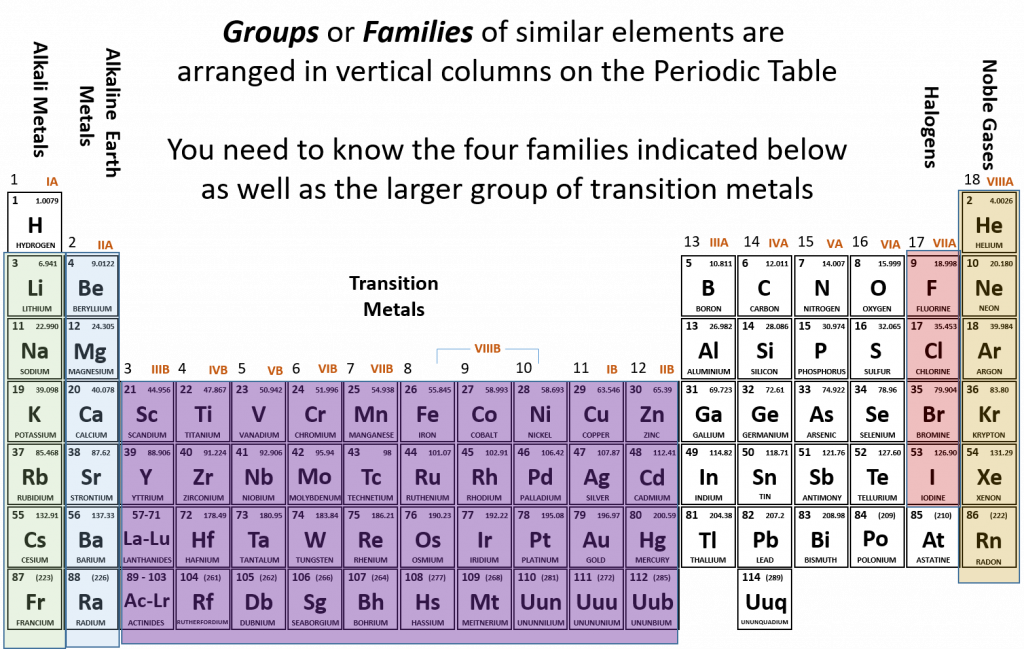
2.6 Families of the Periodic Table
Remember that Mendeleev arranged the periodic table so that elements with the most similar properties were placed in the same group. A group, or family of elements, is a vertical column of the periodic table. Elements are placed into families due to their similar properties, characteristics, and reactivity. For example, all of the elements in group 1 (except for hydrogen, which has unique properties) are very reactive and form compounds in the same ratios and with similar properties as other 1 elements. Due to the similarities in their chemical properties, Mendeleev put these elements into the same group and they came to be known as the alkali metals. The alkali metals include: lithium, sodium, potassium, rubidium, cesium, and francium. Alkali metals are among the most reactive metals. This is due in part to their larger atomic radii and low ionization energies, that will be discussed in more details in section 2.8 below. They get their name from ancient Arabic (al qali) because “scientists” of the time found that the ashes of the vegetation they were burning contained a large amount of sodium and potassium. In Arabic, al qali means ashes. Although most metals tend to be very hard, alkali metals have a soft texture, are silvery in color and can be easily cut. They also have low boiling and melting points and are less dense than most elements. Figure 2.12 shows some of the most common families on the periodic table.
Figure 2.12 Common Families and Groups of the Periodic Table.
The same pattern is true of other vertical groups on the periodic table. Group 2 is called the alkaline earth metals. Once again these elements have similar properties to each other. alkaline earth metals include Beryllium, Magnesium, Calcium, Barium, Strontium and Radium and are soft, silver metals that are less metallic in character than the Group 1 alkali metals. Although many characteristics are common throughout the group, the heavier metals such as Ca, Sr, Ba, and Ra are almost as reactive as the Group 1 alkali metals. They get their name because early “scientists” found that all of the alkaline earth metals were found in the earth’s crust.
The transition metals are the larger block of elements shown in purple on Figure 2.12 extending from Groups 3-12 (also known as the group B elements). Transition elements differ from the main group elements (group A elements) in that they tend to be hard and have high densities. They have high melting points and boiling points and can show various oxidation states when forming chemical bonds (this will be discussed further in chapter 3). They often form colored compounds that are highly stable and they can serve as good catalysts. A catalyst is an agent that helps to speed up a chemical reaction without itself being changed in the process.
Group 17 elements are also called halogens. This group contains very reactive nonmetals. The halogens are an interesting group. Halogens are members of Group 17, which is also referred to as 7A. It is the only group in the Periodic Table that contains all of the states of matter at room temperature. Fluorine, F2 and chlorine, Cl2 are gases, while Bromine, Br2, is a liquid and iodine, I2, and astatine, At2, are both solids. Another interesting feature about Group 17 is that it houses four (4) of the seven (7) diatomic elements. Diatomic elements only exist in nature as a pair of atoms of the same element that are bonded together. The seven diatomic elements are H2, N2, O2, F2, Cl2, Br2, and I2. Notice that the latter four are Group 17 elements. The word halogen comes from the Greek meaning salt forming. French chemists discovered that the majority of halogen ions will form salts when combined with metals.
The noble gases are in group 18. The two most significant properties of noble gases is that they are extremely unreactive, rarely forming compounds, and that they all exist as gases at room temperature. We will learn the reason for their unreactivity when we discuss how compounds form in chapters 3 and 4. The first person to isolate a noble gas was Henry Cavendish, who isolated argon in the late 1700s. The noble gases were actually considered inert gases until the 1960s when a compound was formed between xenon and fluorine which changed the way chemists viewed the “inert” gases. In the English language, inert means to be lifeless or motionless; in the chemical world, inert means does not react. Later, the name “noble gas” replaced “inert gas” for the name of Group 18. The elements in this group are also gases at room temperature.
2.7 Defining the Atom
Basic Atomic Structure – electrons, neutrons, and protons
The modern atomic theory, proposed about 1803 by the English chemist John Dalton, is a fundamental concept that states that all elements are composed of atoms. An atom is the smallest part of an element that maintains the identity of that element. Individual atoms are extremely small; even the largest atom has an approximate diameter of only 5.4 × 10−10 m. With that size, it takes over 18 million of these atoms, lined up side by side, to equal the width of your little finger (about 1 cm).
Most elements in their pure form exist as individual atoms. For example, a macroscopic chunk of iron metal is composed, microscopically, of individual iron atoms. Some elements, however, exist as groups of atoms called molecules. Several important elements exist as two-atom combinations and are called diatomic molecules. In representing a diatomic molecule, we use the symbol of the element and include the subscript 2 to indicate that two atoms of that element are joined together. The elements that exist as diatomic molecules are hydrogen (H2), oxygen (O2), nitrogen (N2), fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2).
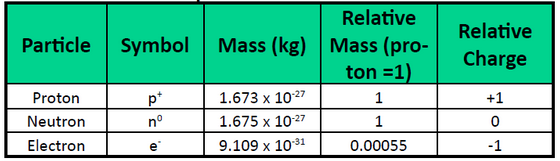
Atoms are made up of extremely small subatomic particles called protons, neutrons, and electrons. Protons are positively charged particles with a relative mass of 1.672622×10-24g, which form part of the core nucleus of an atom. The other part of the atomic nucleus is made up of neutrons, electrically neutral particles with a relative mass almost identical to a proton (1.674927×10-24g). Electrons are extremely small (9.109328×10-28g) negatively charged particles that form an electron cloud, which orbits the nucleus. Table 1.4 summarizes some of the general properties of subatomic particles.
Table 1.4 Properties of Subatomic Particles
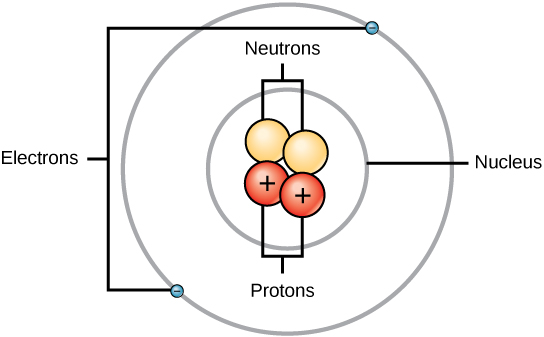
Experiment have shown that protons and neutrons are concentrated in a central region of each atom called the nucleus (plural, nuclei). Electrons are outside the nucleus and orbit about it because they are attracted to the positive charge in the nucleus. Figures 2.13 and 2.14 depict the structure of an atom.
Figure 2.13 The Anatomy of an Atom. The protons and neutrons of an atom are found clustered at the center of the atom in a structure called the nucleus. The electrons orbit the nucleus of the atom within an electron cloud, or the empty space that surrounds the atom’s nucleus. Note that most of the area of an atom is taken up by the empty space of the electron cloud.
Diagram provided by: CNX OpenStax
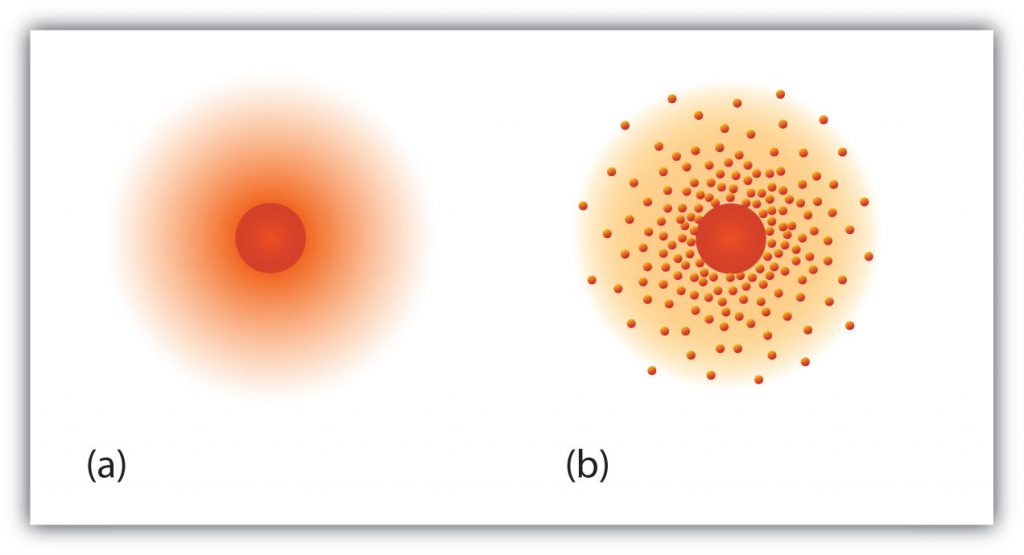
Fig 2.14 The path of the electron in a hydrogen atom. Electrons are not in discrete orbits like planets around the sun. Instead there is a probability that an electron may occupy a certain space within the electron cloud (a) The darker the color, the higher the probability that the hydrogen’s one electron will be at that point at any given time. (b) Similarly, the more crowded the dots, the higher the probability that hydrogen’s one electron will be at that point. In both diagrams, the nucleus is in the center of the diagram.
The electrons within an atom are arranged within specific energy shells. These shells can also be thought of as electron clouds, as the electrons are in constant motion around the nucleus of the atom. Notably each period on the periodic table (ie rows n1-n7) represents the number of electron shells present in the elements within that period. For example, Aluminum is in the 3rd row of the periodic table and, thus, contains a total of 3 electron shells. Shells that are positioned more closely to the nucleus are of lower energy than shells that are farther away (ie shell n1 is the closest to the nuclei and will have the lowest energy levels). Within each shell the electrons are further divided into subshells and then orbitals within each subshell. With regards to chemical bonding, the most important electrons within the atom are known as the valence electrons. The valence electrons are the electrons that are located at the outermost edge of the atom. For almost all of the elements on the periodic table, the maximum number of valence electrons within an atom is eight. This is known as the octet rule. In Chapter 4, we will begin to see how valence electrons within atoms are involved in chemical bonding.
Interestingly, most of the mass of an atom is housed in the nucleus of the atom, held in the protons and the neutrons. However, the size of the nucleus is very, very tiny in relation to the entire atom. Most of the space within an atom is made up of the electron cloud. For example, if an atom was the size of a soccer stadium, the nucleus would be about the size of a small marble at the center of the stadium. The rest of the stadium would represent the empty space of the electron cloud. As a result, an atom consists largely of empty space.
Atomic particles are so small that it is impractical to measure them in grams, instead we use a relative mass scale which makes the numbers much more manageable. We use Atomic Mass Units (AMU or u) to measure the mass of atomic particles, one AMU is equal to 1/12th the mass of an atom of carbon-12. You may also see Atomic Mass Units referred to as Daltons (Da) after John Dalton, the English Chemist that first proposed the atomic theory. Carbon-12 has 6 protons and 6 neutrons in its nucleus, meaning that one amu is equal to the average of the masses of a proton and a neutron. The mass of an atom in AMUs is equal to the number of protons and neutrons making up the atom. For example the atomic mass of bromine is roughly 80 amu and its proton number is 35, meaning that bromine has 35 protons and 45 neutrons in its nucleus.
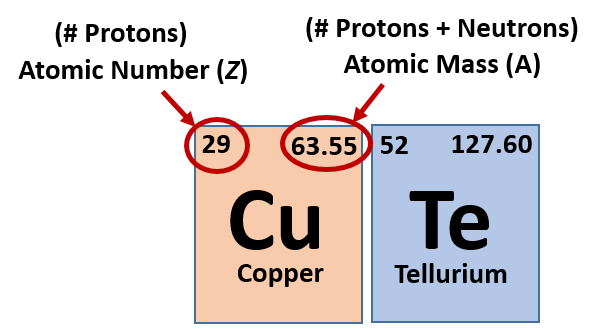
2.8 Atomic Number – Protons Determine the Identity of an Element
When looking at the periodic table you might notice that for each element there are two sets of numbers around the symbol. These symbols correspond to important values that give you important information about each element (Figure 2.15). The most important value corresponding to characteristics of an element is the proton number, which is also called atomic number (represented by the mathematical term, Z). As it turns out, the number of protons that an atom holds in its nucleus is the key determining feature for its chemical properties. In short, an element is defined by the number of protons found in its nucleus. If you refer back to the Periodic Table of Elements shown in figure 2.10, you will see that the periodic table is organized by the number of protons that an element contains. Thus, as you read across each row of the Periodic Table (left to right), each element increases by one proton (or one Atomic Number, Z). When atoms are in their elemental states, their overall charge is zero and the atoms are neutral. Since protons are positively charged and electrons are negatively charged, this means that when atoms are in their elemental form, the number of protons equals the number of electrons. Therefore, if you know the atomic number of an atom, you also know how many electrons are present in that atom when it is in its elemental form. When atoms combine with one another to form compounds, like water (H2O), they will either share or donate/accept electrons from their bonding partners. It is this movement of electrons that facilitates chemical bond formation. Thus, during bond formation the number of electrons around an atom may change, but the atomic number (or number of protons) remains constant and does not change.
Fig 2.15 Structure of the Periodic Table. Each element on the periodic table is represented by the atomic symbol (Cu for Copper, and Te for Tellurium). Sometimes the Atomic Number is written in the upper lefthand corner, and the Atomic Mass in the righthand corner, as shown in this figure. Sometimes, periodic tables will show the atomic number above the element symbol and the atomic mass below the element symbol, as shown in the periodic table in Figure 2.10.
2.9 Atomic Mass, Isotopes, and Molar Mass
Atomic Mass
Atomic mass (A) is the total mass of an atom of a specific element and can be calculated by adding up the number of protons and neutrons present within an atom. The electrons are ignored in the mass calculation because they are so small that they barely add any mass to the atom.
# Protons + # Neutrons = Atomic Mass
Thus, if you know any two of the the three values (atomic mass, atomic number, or number of neutrons), you can calculate the third value. For example, nitrogen has an atomic mass of 14.007 and an atomic number of 7. Thus, it contains 7 protons, and 7 neutrons (14.007 – 7 = 7.007, which is then rounded to 7). Note that the number of neutrons in an atom does not have to equal the number of protons in the atom. For example, lead (Pb), contains 82 protons and has an atomic mass of 207.2. Thus it contains 125 neutrons (207.2 – 82 = 125). Thus, if you know the atomic mass and the atomic number of an element, you can calculate the number of neutrons present, or if you know the atomic mass and the number of neutrons, you can calculate the atomic number.
Isotopes
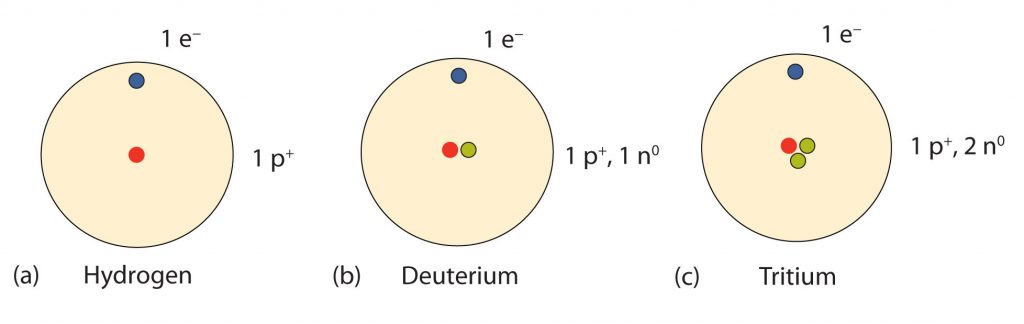
So…how many neutrons are in atoms of a particular element? At first it was thought that the number of neutrons in a nucleus was also defining characteristic of an element. However, it was found that atoms of the same element can have different numbers of neutrons. Atoms of the same element that have different numbers of neutrons are called isotopes. An example of the three common isotopes of hydrogen are shown in Figure 2.16. Note that each of the hydrogen isotopes is known by a unique name, hydrogen, deuterium, and tritium. Not all elemental isotopes have such unique names. Many of the isotopes are distinguished from one another by including the atomic mass in the definition. For example, 99% of the carbon atoms on Earth have 6 neutrons and 6 protons in their nuclei, this is known as carbon-12; just under 1% of the carbon atoms have 7 neutrons and 6 protons in their nuclei, which is known as carbon-13, and an even smaller percent is carbon with 8 neutrons and 6 protons, or carbon-14. Carbon-14 is unstable and will decay over time making it a radioactive form of carbon. The characterization of radioactive materials will be covered in more detail in Chapter 3. Overall, there are 15 known isotopes of carbon! Thus, naturally occurring carbon on Earth, therefore, is actually a mixture of isotopes, albeit a mixture that is 99% carbon-12. Isotope composition has proven to be a useful method for dating many rock layers and fossils.
Fig 2.16 Isotopes of Hydrogen. All hydrogen atoms have one proton and one electron. However, they can differ in the number of neutrons. (a) Most hydrogen atoms only contain one proton and one electron and no neutrons (b) A small amount of hydrogen exists as the isotope deuterium, which has one proton and one neutron in its nucleus, and (c) an even smaller amount contains one proton and two neutrons in its nucleus and is termed Tritium. Note that Tritium is unstable isotope and will breakdown over time. Thus, Tritium is a radioactive element.
Most elements exist as mixtures of isotopes. In fact, there are currently over 3,500 isotopes known for all the elements. When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. A simple way of indicating the mass number of a particular isotope is to list it as a superscript on the left side of an element’s symbol. Atomic numbers are often listed as a subscript on the left side of an element’s symbol. Thus, we might see
which indicates a particular isotope of copper. The 29 is the atomic number, Z, (which is the same for all copper atoms), while the 63 is the atomic mass (A) of the isotope. To determine the number of neutrons in this isotope, we subtract 29 from 63: 63 − 29 = 34, so there are 34 neutrons in this atom.
The atomic masses indicated on the periodic table represents an average mass for each element based on the proportion of each isotope present on the Earth. This is why most of the atomic masses on the periodic table are not exact numbers. For example, the atomic mass of copper is 63.546 amu. This mass is an average of an element’s atomic masses, weighted by the natural abundance of each isotope. So how could we calculate atomic mass based on the natural abundance of different isotopes of an element?
Example: Calculating Atomic Mass Using Isotope Abundance
Extra Practice:
Try to work out the atomic mass for boron. Boron exists as a mixture that is 19.9% 10B and 80.1% 11B. Calculate the atomic mass. Check the periodic table for the correct answer!
Molar Mass
In Chapter 1, you learned that 1 mole of any substance is equivalent to 6.02 X 1023 molecules of that substance. But why this number? It seems like a strange number for chemists to work with! However, it turns out that this number of items of any atom or any substance is proportional to the atomic mass of that substance in grams, and this is very useful indeed! Atoms and molecules are way too small to count on an individual scale. However, when you are setting up a chemical reaction in the laboratory, reactions are written such that you know how many of each reactant will react with the other reactant to give you a known number of product molecules. It would be very expensive and wasteful indeed, if we did not have some way to determine how many molecules that we are adding of each substance when we are conducting a chemical reaction. Thus, the relationship of Avogadro’s Number to the Molar Mass of a substance provides us with a mechanism to convert the number of molecules present into their gram mass. It is easily possible for us to measure out gram quantities of substances in the laboratory using a simple balance. Thus, by using the relationship of Avogadro’s Number with the Molar Mass, we are able to conduct very precise measurements of the substances used in chemical reactions, saving both time and money!
So how does it work? We just learned in the section above that the atomic mass of each element is given on the periodic table, and is determined by the number of protons and neutrons present in the atom. For example the atomic mass of Sodium (Na) is 22.990 Daltons or Atomic Mass Units. If we were to relate this mass to the mass of 1 mole of Na atoms, the mass would be 22.990 grams. This would mean that 6.02 x 1023 atoms of Sodium has a mass of 22.990 g! Similarly, for any substance, such as water (H2O), we need only to add the atomic masses of each element in the substance together to obtain the molar mass of that substance. For water, there are 2 Hydrogens and 1 Oxygen atom. Adding the atomic masses of these elements together, we would get 2(1.0079) + 15.999 = 18.0148 atomic mass units. This is then equivalent to the molar mass of water which is 18.0148 g of water = 1 mole of water = 6.02 x 1023 molecules of water. Thus, the overall definition of molar mass is that the molar mass of a substance is equal to the atomic mass of that substance in grams and is equivalent to 6.02 x 1023 molecules of that substance.
A little practice:
- What is the molar mass of glucose (C6H12O6)?
- What is the molar mass of Sodium Hydrogen Carbonate (NaHCO3)?
- How many moles is 42.3 grams of Magnesium (Mg)?
- How many atoms are in 63 grams of Mercury (Hg)?
2.10 Periodic Table Trends
Now that you have learned some basic concepts about atomic structure and the organization of elements on the periodic table, we can use that knowledge to discuss some common periodic trends.
Atomic Size
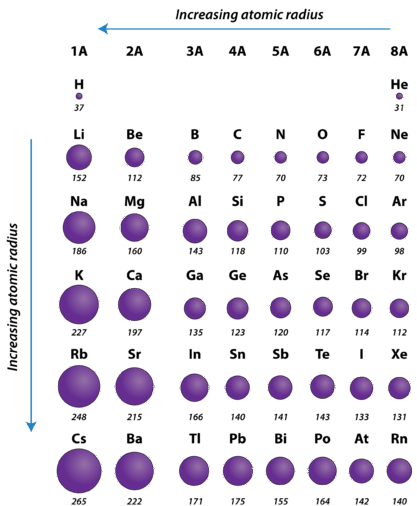
One important trend to be aware of is the way that atomic size changes as you move across a period or down a group in the periodic table. Atomic size is typically measured by the radius of the atom starting at the core of the nucleus, and reaching all the way out to the last valence electron. As you may predict, atomic size will increase as you move down a family group, due to the increased number of electron shells. This substantially increases the size of the electron cloud. What you may not so easily predict is that atomic size decreases as you go across a period. Although this may seem counter-intuitive, the decrease in size can be explained by thinking about the valence electron shell as you go across a period; we see that each element across a period has the same valence electron shell that it is filling with valence electrons. While the valence shell stays the same as you go across a period, the number of protons and electrons is increasing. Protons, being positively charged, have a pull on the negatively charged electrons out in the electron cloud. As the number of protons and electrons increases across a period, they have an attractive pull on one another. This results in a tightening of the electron cloud and a reduction in the atomic nuclei. In other words, because the outermost electron shell remains the same across a period, that shell gets pulled progressively closer and closer to the nucleus of the atom as you go across a period. So the overall periodic trend for atomic radius (size) is that atoms get smaller as you go across a period, and they get larger as you go down a family group (Figure 2.16).
Figure 2.17 Atomic Radii of Select Elements Across the Periodic Table
Overall:
- Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together.
- The atomic radius of atoms generally decreases from left to right across a period.
- The atomic radius of atoms generally increases from top to bottom within a group.
Electronegativity
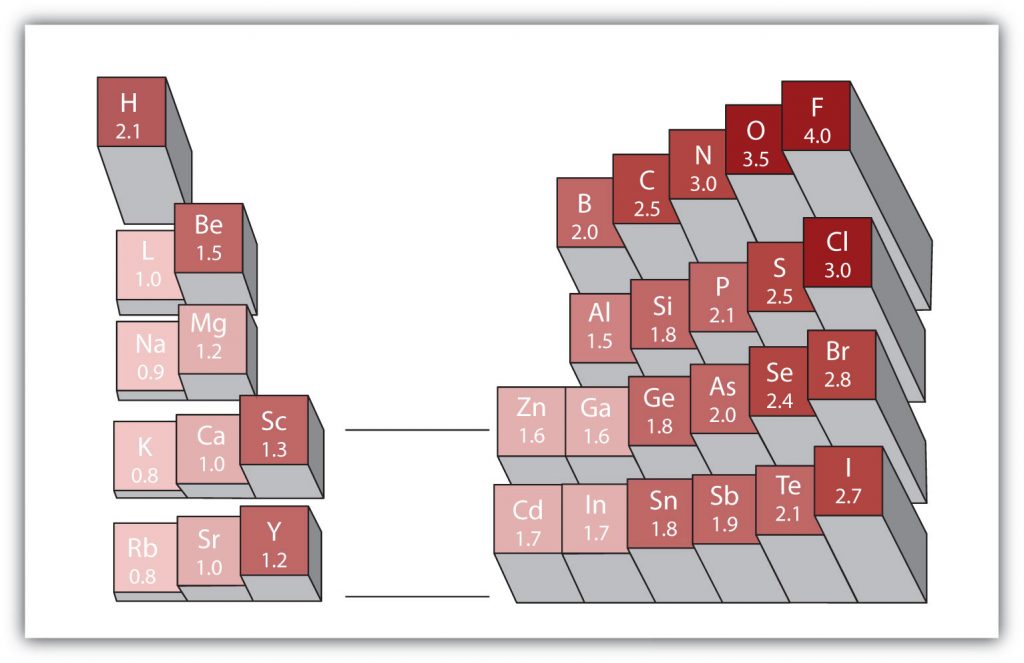
Another important periodic trend to be aware of is how electronegativity differences can be identified. Electronegativity is the measure of an atom’s tendency to attract a bonding pair of electrons. There are various numerical scales for rating electronegativity. Figure 2.18 shows one of the most popular—the Pauling scale. The Pauling scale assigns fluorine, the most electronegative atom, a 4.0 while less electronegative atoms have smaller grades. We will see in chapters 3 and 4 that electronegativity plays an important role in chemical bonding. The trends for electronegativity in the periodic table are that electronegativity increases as you go across a period, and increases as you go up a group, with fluorine being the most electronegative atom. Noble gases are given an electronegativity rating of 0 due to their inherent stability, which keeps them from forming bonds with other atoms.
Figure 2.18 Electronegativities of Various Elements. The Pauling Scale for electronegativities has the value for fluorine atoms set at 4.0, the highest value.
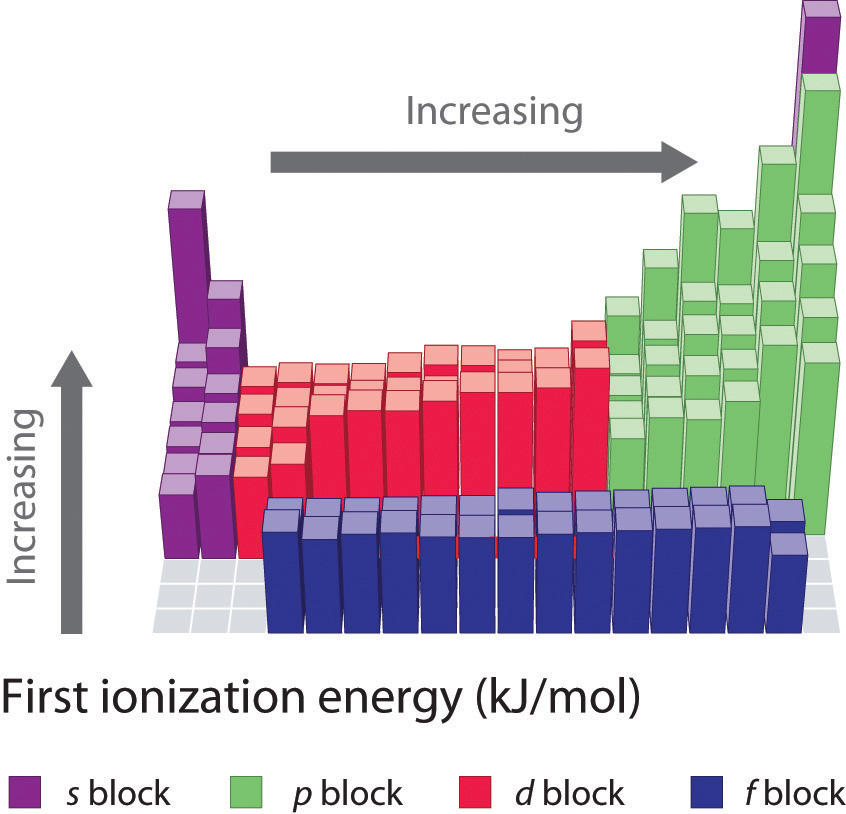
Ionization Energy
Another periodic trend that you will be expected to know is the trend for ionization energy. Ionization energy is defined as the amount of energy required to remove the most loosely bound electron of an atom. How tightly bound the electrons of an atom are will affect the amount of energy required to remove one of the valence electrons. Electrons that are closer to the nucleus are going to be more tightly held than those that are further away and will require more energy to pull them off of the atom. For this reason, we see that ionization energy decreases as you go down a family group and the atoms get larger. This same concept can be applied to atoms across a period. We will see that the highest ionization energy will be found on the right side of the period where the atoms are the smallest, and the lowest ionization energy on the left where the atoms have a larger radii. In general, ionization energy decreases as you go down a family group, and increases as you go across a period from left to right (Figure 2.19).
Figure 2.19 First Ionization Energies for the Elements of the Periodic Table.
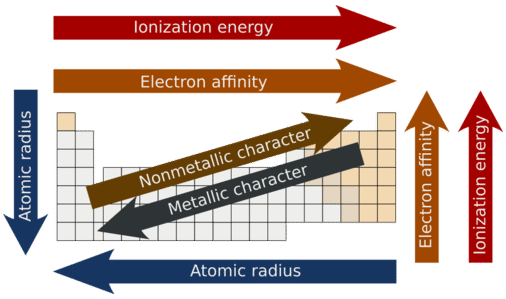
Metallic and Nonmetallic Character
Metallic character refers to the level of reactivity of a metal. Metals tend to lose electrons in chemical reactions, as indicated by their low ionization energies. Within a compound, metal atoms have relatively low attraction for electrons, as indicated by their low electronegativities. By following the trend summary in the figure below, you can see that the most reactive metals would reside in the lower left portion of the periodic table. The most reactive metal is cesium, which is not found in nature as a free element. It reacts explosively with water and will ignite spontaneously in air. Francium is below cesium in the alkali metal group, but is so rare that most of its properties have never been observed.
The metallic character increases as you go down a group. Since the ionization energy decreases going down a group (or increases going up a group), the increased ability for metals lower in a group to lose electrons makes them more reactive. In addition, the atomic radius increases going down a group, placing the outer electrons further away from the nucleus and making that electron less attracted by the nucleus.
Nonmetals tend to gain electrons in chemical reactions and have a high attraction for electrons within a compound. These tendencies are known as nonmetallic character. The most reactive nonmetals reside in the upper right portion of the periodic table. Since the noble gases are a special group because of their lack of reactivity, the element fluorine is the most reactive nonmetal. It is not found in nature as a free element. Fluorine gas reacts explosively with many other elements and compounds and is considered to be one of the most dangerous known substances.
Note that there is no clear division between metallic and nonmetallic character. As we move across the periodic table, there is an increasing tendency to accept electrons (nonmetallic) and a decrease in the possibility that an atom would give up one or more electrons.
Overall
- Metallic character refers to the level of reactivity of a metal to donate electrons during a chemical reaction.
- Nonmetallic character relates to the tendency of an element to accept electrons during chemical reactions.
- Metallic character increases going down a family group and decreases going across a period.
- Nonmetallic character increases going from left to right across the periodic table and decreases going down a family group.
The following figure summarizes all of the major periodic trends within the periodic table
Figure 2.20 Summary of Major Periodic Trends
2.10 Chapter Summary
Dalton’s Atomic Theory proposed that matter is made up of tiny particles called atoms that cannot be broken into smaller pieces. During a chemical reaction, atoms are rearranged, but they do not break apart, nor are they created or destroyed. An element is a substance that cannot be broken down into simpler chemical substances. There are about 90 naturally occurring elements known on Earth. The smallest unit of an element is the atom. All atoms of the same element are identical in mass and other properties, whereas atoms of different elements differ in mass and other properties.
The elements can be divided into three major classes: The metals, metalloids, and nonmetals. Metals are typically shiny, very dense, have high melting points, and are good conductors. Nonmetals are generally brittle, dull, have low melting points, and they are generally poor conductors. Metalloids have properties of both metals and nonmetals.
Dmitri Mendeleev organized the elements into a chart based on their similar characteristics and properties. Today this chart is known as the periodic table of the elements. Within the periodic table group, or family of elements, is a vertical column of the periodic table. Elements are placed into families due to their similar properties, characteristics, and reactivities. Group 1 elements are known as the alkali metals and are the most reactive elements of the metal class. Alkaline earth metals are found in group 2 and are almost as reactive as the group 1 metals. The transition metals are the larger block of elements extending from Groups 3-12 (also known as the group B elements). Transition metals have high melting points and boiling points, often form colored compounds that are highly stable, and they can serve as good catalysts. A catalyst is an agent that helps to speed up a chemical reaction without itself being changed in the process. Group 17 elements, known as halogens, contains very reactive nonmetals that often exist as diatomic elements (F2, Cl2, Br2, I2). Group 18 elements, the noble gases are extremely stable, unreactive, and rarely form compounds.
Atoms are made up of extremely small subatomic particles called protons, neutrons, and electrons. Protons (positively charged particles), and neutrons (electrically neutral particles) form the core or nucleus of an atom. Electrons are extremely small, negatively charged particles that form an electron cloud, which orbits the nucleus. The atomic number (Z) refers the the number of protons present in an element and is the defining feature of an element. The atomic mass (A) of an element is the sum of the protons and neutrons within that element.
Atoms of the same element (have the same atomic number) that have different numbers of neutrons are called isotopes. Most elements exist as isotopes. In fact, over 3,500 isotopes are known for the different elements. The atomic mass reported on the periodic table is the relative mass of the different isotopes of an element based on their abundance on the Earth.
The valence shell of an element is the outermost electron shell. The electrons housed within the valence shell are the most reactive electrons in an atom and are essential for forming chemical bonds with other elements. There are a total of eight electrons that can be housed in the valence shell of any atom.
Due to the organization of the periodic table according to proton and electron configurations, a number of interesting elemental trends can be observed. Atomic size, as measured by the atomic radius of an atom, increases and you move down a family group, and decreases as you move from left to right down a period or a row on the periodic table. The Electronegativity of an atom is the measure of an atom’s tendency to attract a bonding pair of electrons, and can be thought of as electron affinity. Electronegativity increases as you go from left to right across the periods of the periodic table and it tends to decrease as you move down family groups. Ionization energy is defined as the amount of energy required to remove the most loosely bound electron of an atom. Ionization energy tends to increase as you move across the periods of the periodic table from left to right, and decreases as you move down a family group. Metallic character refers to the level of reactivity of a metal, whereas nonmetallic character refers to the level of reactivity of nonmetals. The metallic character of the elements tends to go up as you move down a family group of elements and goes down as you move from left to right across a row of the periodic table. Concomitantly, nonmetallic character tends to go down as you move down a family group of elements and goes up as you move from the left to the right across the periodic table.
Homework Chapter 2
Part 1: Atomic Structure
2.11 References
Chapter 2 materials have been adapted and modified from the following creative commons resources unless otherwise noted:
1. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
2. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
3. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12.
4. LibreTexts (2017) Chem 121: Chapter 2 Atomic Structure. https://chem.libretexts.org/LibreTexts/Valley_City_State_University/Chem_121/Chapter_2%3A_Atomic_Structure/2.03%3A_Families_and_Periods_of_the_Periodic_Table
5. Robson, G.(2006) Wikipedia. https://en.wikipedia.org/wiki/Electron_shell
6. LibreTexts (2016) Physical and Theoretical Chemistry. https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Theory/Electrons_in_Atoms/Electronic_Orbitals
7. High School Chemistry/Families on the Periodic Table. (2016) Wikibooks. https://en.wikibooks.org/wiki/High_School_Chemistry/Families_on_the_Periodic_Table#Alkali_Metals_Have_One_Electron_in_Their_Outer_Energy_Level
8. 1. Anonymous. (2012) Principles of General Chemistry (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://webmis.highland.cc.il.us/~jsullivan/principles-of-general-chemistry-v1.0/index.html
9. Key, J. (2018) Radioactivity. Published under Creative Commons by-nc-sa 4.0. Available at: https://opentextbc.ca/introductorychemistry/chapter/radioactivity-2/
10. Rice University () Biological Effects of Radiation. Published under Creative Commons by-nc-sa 4.0. Available at: https://opentextbc.ca/chemistry/chapter/21-6-biological-effects-of-radiation/