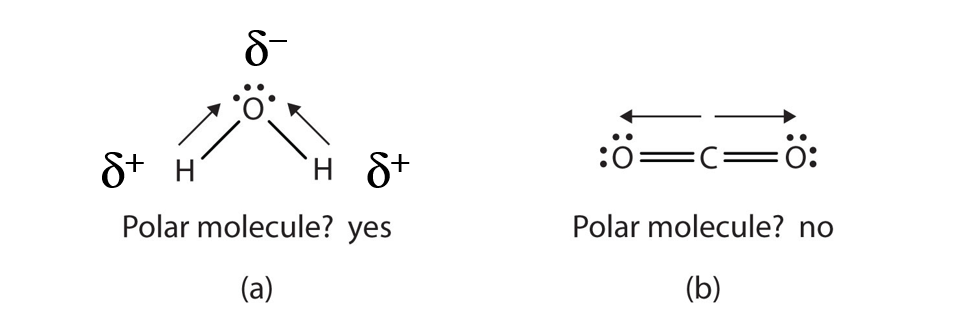Home » Student Resources » Online Chemistry Textbooks » CH150: Preparatory Chemistry » CH150: Chapter 4 – Covalent Bonds and Molecular Compounds
MenuCH150: Preparatory Chemistry
Chapter 4 – Covalent Bonds and Molecular Compounds
This text is published under creative commons licensing, for referencing and adaptation, please click here.
4.1 Introduction to Covalent Molecules and Compounds
How to Recognize Covalent Bonds
4.2 Electron Sharing
Single Covalent Bonds Between the Same Atoms
Single Covalent Bonds Between Different Atoms
Multiple Covalent Bonds
Coordinate Covalent Bonds
4.3 Electronegativity and Bond Polarity
4.4 Properties of Molecular Compounds
4.5 Naming Binary Molecular Compounds
4.6 Chapter Summary
4.7 References
Chapter 4 – Covalent Bonds and Molecular Compounds
Chemical bonds are generally divided into two fundamentally different types: ionic and covalent. In reality, however, the bonds in most substances are neither purely ionic nor purely covalent, but lie on a spectrum between these extremes. Although purely ionic and purely covalent bonds represent extreme cases that are seldom encountered in any but very simple substances, a brief discussion of these two extremes helps explain why substances with different kinds of chemical bonds have very different properties. Ionic compounds consist of positively and negatively charged ions held together by strong electrostatic forces, whereas covalent compounds generally consist of molecules, which are groups of atoms in which one or more pairs of electrons are shared between bonded atoms. In a covalent bond, atoms are held together by the electrostatic attraction between the positively charged nuclei of the bonded atoms and the negatively charged electrons they share. This chapter will focus on the properties of covalent compounds.
4.1 Introduction to Covalent Molecules and Compounds
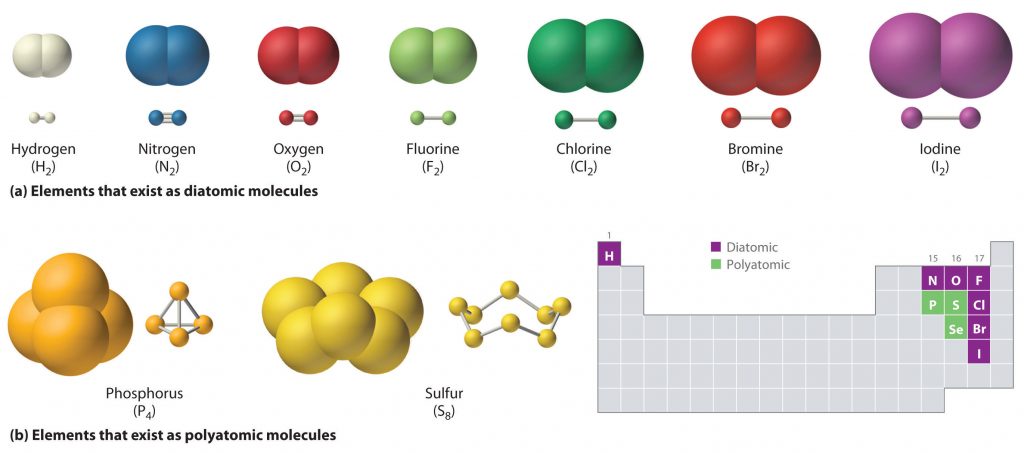
Just as an atom is the simplest unit that has the fundamental chemical properties of an element, a molecule is the simplest unit that has the fundamental chemical properties of a covalent compound. Thus, the term molecular compound is used to describe elements that are covalently bonded and to distinguish the compounds from ionic compounds. Some pure elements exist as covalent molecules. Hydrogen, nitrogen, oxygen, and the halogens occur naturally as the diatomic (“two atoms”) molecules H2, N2, O2, F2, Cl2, Br2, and I2 (part (a) in Figure 4.1). Similarly, a few pure elements exist as polyatomic (“many atoms”) molecules, such as elemental phosphorus and sulfur, which occur as P4 and S8 (part (b) in Figure 4.1).
Figure 4.1 Elements That Exist as Covalent Molecules. (a) Several elements naturally exist as diatomic molecules, in which two atoms (E) are joined by one or more covalent bonds to form a molecule with the general formula E2. (b) A few elements naturally exist as polyatomic molecules, which contain more than two atoms. For example, phosphorus exists as P4 tetrahedra—regular polyhedra with four triangular sides—with a phosphorus atom at each vertex. Elemental sulfur consists of a puckered ring of eight sulfur atoms connected by single bonds. Selenium is not shown due to the complexity of its structure.
Each covalent compound is represented by a molecular formula, which gives the atomic symbol for each component element, in a prescribed order, accompanied by a subscript indicating the number of atoms of that element in the molecule. The subscript is written only if the number of atoms is greater than 1. For example, water, with two hydrogen atoms and one oxygen atom per molecule, is written as H2O. Similarly, carbon dioxide, which contains one carbon atom and two oxygen atoms in each molecule, is written as CO2.
Covalent compounds that predominantly contain carbon and hydrogen are called organic compounds. The convention for representing the formulas of organic compounds is to write carbon first, followed by hydrogen and then any other elements in alphabetical order (e.g., CH4O is methyl alcohol, a fuel). Compounds that consist primarily of elements other than carbon and hydrogen are called inorganic compounds; they include both covalent and ionic compounds. The convention for writing inorganic compounds, involves listing the component elements beginning with the one farthest to the left in the periodic table, as in CO2 or SF6. Those in the same group are listed beginning with the lower element and working up, as in ClF. By convention, however, when an inorganic compound contains both hydrogen and an element from groups 13–15, hydrogen is usually listed last in the formula. Examples are ammonia (NH3) and silane (SiH4). Compounds such as water, whose compositions were established long before this convention was adopted, are always written with hydrogen first: Water is always written as H2O, not OH2. Typically this distinguishes when hydrogen is participating in a covalent bond rather than an ionic interaction, as seen in many of the inorganic acids, such as hydrochloric acid (HCl) and sulfuric acid (H2SO4), as described in chapter 3.
How to Recognize Covalent Bonds
In Chapter 3, we saw that ionic compounds are composed predominantly of a metal + a nonmetal. Covalent molecules, on the otherhand, are typically composed of two nonmetals or a nonmetal and a metalloid. This is an initial screening method that you can use to categorize compounds into the ionic or the covalent cagetogy.
Figure 4.2 Recognizing Ionic vs Covalent Compounds. Typically compounds that are formed from a combination of a metal with a nonmetal have more ionic bond character whereas compounds formed from two nonmetals or a metalloid and a nonmetal show more covalent character. Although compounds usually lie on a spectrum somewhere between fully ionic and fully covalent character, for naming purposes, this guideline works well.
4.2 Electron Sharing
Single Covalent Bonds Between the Same Atoms
Chapter 3 described how electrons can be transferred from one atom to another so that both atoms have an energy-stable outer electron shell following the octet rule. However, there is another way an atom can achieve a full valence shell: atoms can share electrons to reach the octet state (or the duet state in the case of hydrogen).
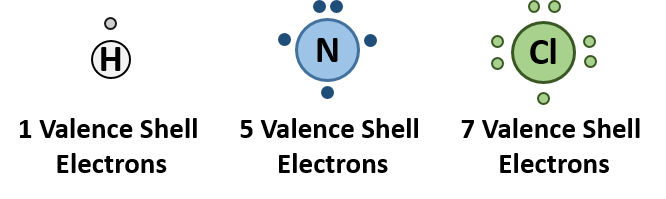
This concept can be illustrated by using two hydrogen atoms, each of which has a single electron in its valence shell. (For small atoms such as hydrogen atoms, the valence shell will be the first shell, which holds only two electrons.) We can represent the two individual hydrogen atoms as follows:
In this situation neither hydrogen can reach the preferred duet state. In contrast, when two hydrogen atoms get close enough together to share their electrons, they can be represented as follows:
By sharing their valence electrons, both hydrogen atoms now have two electrons in their respective valence shells. Because each valence shell is now filled, this arrangement is more stable than when the two atoms are separate. In this configuration, each hydrogen has an electron configuration equivalent to that of the noble gas, helium. The sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are called a bonding pair of electrons. A discrete group of atoms connected by covalent bonds is called a molecule—the smallest part of a compound that retains the chemical identity of that compound. For example, one molecule of water would contain two hydrogen atoms and one oxygen atom (H2O).
Chemists frequently use Lewis electron dot diagrams to represent covalent bonding in molecular substances. For example, the Lewis diagrams of two separate hydrogen atoms are as follows:
The Lewis diagram of two hydrogen atoms sharing electrons looks like this:
This depiction of molecules is simplified further by using a dash to represent a covalent bond. The hydrogen molecule is then represented as follows:
Remember that the dash, also referred to as a single bond, represents a pair of bonding electrons.
The bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10−11 m, or 74 picometers (pm; 1 pm = 1 × 10−12 m). This particular bond length represents a balance between several forces: (1) the attractions between oppositely charged electrons and nuclei, (2) the repulsion between two negatively charged electrons, and (3) the repulsion between two positively charged nuclei. If the nuclei were closer together, they would repel each other more strongly; if the nuclei were farther apart, there would be less attraction between the positive and negative particles.
Fluorine is another element whose atoms bond together in pairs to form diatomic (two-atom) molecules. Two separate fluorine atoms have the following electron dot diagrams:
Each fluorine atom contributes one valence electron, making a single bond and giving each atom a complete valence shell, which fulfills the octet rule:
The circles show that each fluorine atom has eight electrons around it. As with hydrogen, we can represent the fluorine molecule with a dash in place of the bonding electrons:
Each fluorine atom has six electrons, or three pairs of electrons, that are not participating in the covalent bond. Rather than being shared, they are considered to belong to a single atom. These are called nonbonding pairs (or lone pairs) of electrons.
Single Covalent Bonds Between Different Atoms
Now that we have looked at electron sharing between atoms of the same element, let us look at covalent bond formation between atoms of different elements. Consider a molecule composed of one hydrogen atom and one fluorine atom:
Each atom needs one additional electron to complete its valence shell. By each contributing one electron, they make the following molecule:
In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The circles show how the valence electron shells are filled for both atoms (recall that hydrogen is filled with two electrons).
Larger molecules are constructed in a similar fashion, with some atoms participating in more than one covalent bond. For example, water, with two hydrogen atoms and one oxygen atom, and methane (CH4), with one carbon atom and four hydrogen atoms, can be represented as follows:
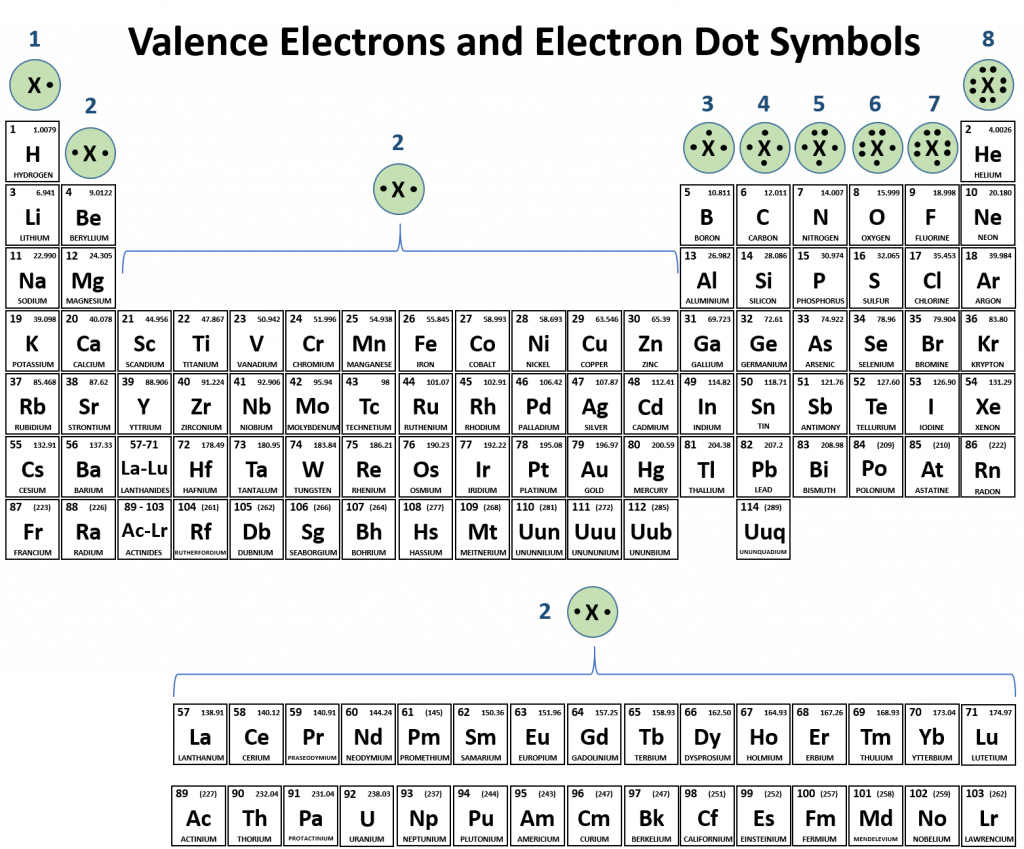
Atoms typically form a characteristic number of covalent bonds in compounds. Figure 4.3 shows valence electron configurations of each element family (or column).
Fig 4.3 Periodic Table with Lewis Structures. Each family shows a representative lewis structure for that group of elements. For the nonmetals (Families 4A, 5A, 6A, and 7A) they can accept a complementary number of shared bonds to reach the octet state. Family 4A can share 4 covalent bonds (4 + 4 = 8), whereas Families 5A, 6A, and 7A can share 3, 2, and 1 covalent bond(s), respectively, to achieve the octet state. Exceptions to the octet rule do exist. For example, hydrogen can be considered to be in Group 1 or Group 7A because it has properties similar to both groups. Hydrogen can participate in either ionic or covalent bonding. When participating in covalent bonding, hydrogen only needs two electrons to have a full valence shell. As it has one electron to start with, it can only make one covalent bond. Similarly, boron has 3 electrons in its outer shell. This nonmetal typically forms 3 covalent bonds, having a maximum of 6 electrons in its outer shell. Thus, boron can never reach the octet state. Other atoms can have expanded orbitals and accept additional covalent bonds. Two of these that are important for living systems are sulfur and phosphorus. By the octet rule, sulfur can make 2 covalent bonds and phosphorus 3 covalent bonds. Sulfur can also have expanded orbitals to accept 4 or 6 covalent bonds, and phosphorus can expand to 5 covalent bonds.
Multiple Covalent Bonds
In many molecules, the octet rule would not be satisfied if each pair of bonded atoms shares only two electrons. Consider carbon dioxide (CO2). If each oxygen atom shares one electron with the carbon atom, we get the following:
This does not give either the carbon or oxygen atoms a complete octet; The carbon atom only has six electrons in its valence shell and each oxygen atom only has seven electrons in its valence shell. Thus, none of the atoms can reach the octet state in the current configuration. As written, this would be an unstable molecular conformation.
Sometimes more than one pair of electrons must be shared between two atoms for both atoms to have an octet. In carbon dioxide, a second electron from each oxygen atom is also shared with the central carbon atom, and the carbon atom shares one more electron with each oxygen atom:
In this arrangement, the carbon atom shares four electrons (two pairs) with the oxygen atom on the left and four electrons with the oxygen atom on the right. There are now eight electrons around each atom. Two pairs of electrons shared between two atoms make a double bond between the atoms, which is represented by a double dash:
Some molecules contain triple bonds, covalent bonds in which three pairs of electrons are shared by two atoms. A simple compound that has a triple bond is acetylene (C2H2), whose Lewis diagram is as follows:
Coordinate Covalent Bonds
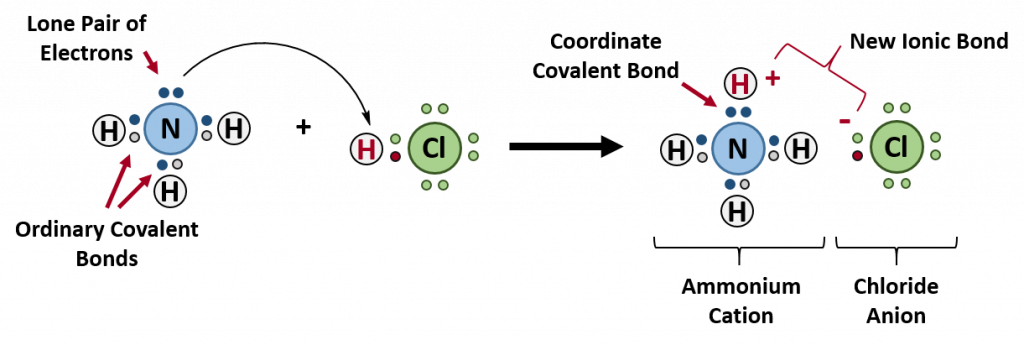
A coordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom. A covalent bond is formed by two atoms sharing a pair of electrons. The atoms are held together because the electron pair is attracted by both of the nuclei. In the formation of a simple or ordinary covalent bond, each atom supplies one electron to the bond – but that does not have to be the case. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons. The following reaction between ammonia and hydrochloric acid demonstrates the formation of a coordinate covalent bond between ammonia and a hydrogren ion (proton).
The reaction between ammonia and hydrochloric acid
If these colorless gases are allowed to mix, a thick white smoke of solid ammonium chloride is formed.
The overall reaction is
Ammonium ions, NH4+, are formed by the transfer of a hydrogen ion (a proton) from the hydrochloric acid molecule to the lone pair of electrons on the ammonia molecule. To visualize this reaction, we can use electron dot configurations to observe the electron movement during the reaction. First recall the valence electron states for all of the atoms involved in the reaction:
On the left side of the equation (to the left of the arrow) are the reactants of the reaction (ammonia and hydrochloric acid). On the right side of the reaction (to the right of the arrow) is the product of the reaction, the ionic compound – ammonium chloride. The diagram below shows the electron and proton movement during the reaction.
Figure 4.4 Formation of Ammonium Chloride. When the ammonium ion, NH4+, is formed, the fourth hydrogen (shown in red) is attached by a coordinate covalent bond, because only the hydrogen’s nucleus is transferred from the chlorine to the nitrogen. The hydrogen’s electron is left behind on the chlorine to form a negative chloride ion. Once the ammonium ion has been formed it is impossible to tell any difference between the coordinate covalent and the ordinary covalent bonds, all of the hydrogens are equivalent in the molecule and the extra positive charge is distributed throughout the molecule. Although the electrons are shown differently in the diagram, there is no difference between them in reality. In simple diagrams, a coordinate bond is shown by a curved arrow. The arrow points from the atom donating the lone pair to the atom accepting it.
4.3 Electronegativity and Bond Polarity
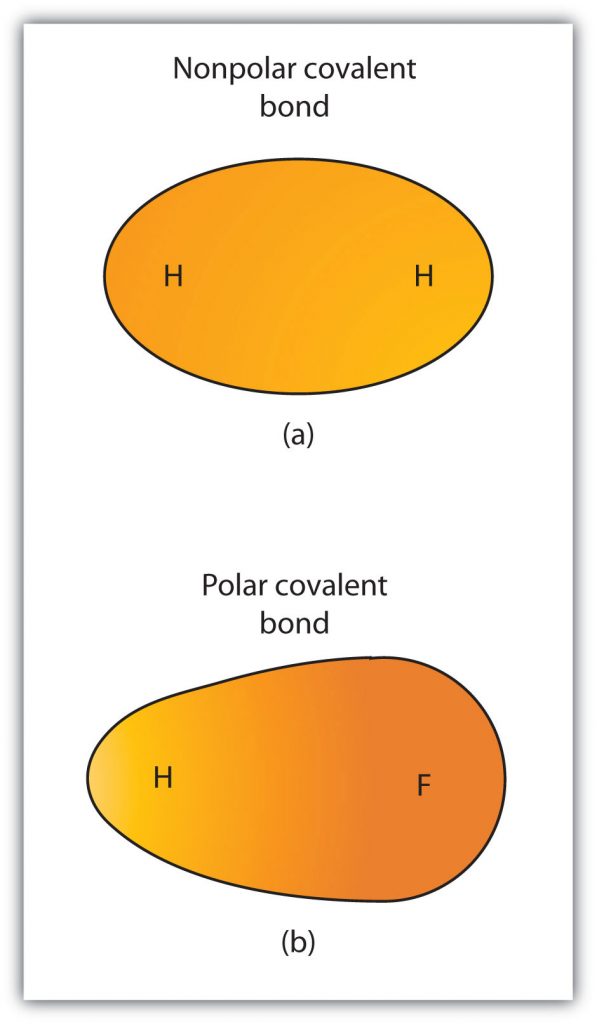
Although we defined covalent bonding as electron sharing, the electrons in a covalent bond are not always shared equally by the two bonded atoms. Unless the bond connects two atoms of the same element, there will always be one atom that attracts the electrons in the bond more strongly than the other atom does, as shown in Figure 4.5. When such an imbalance occurs, there is a resulting buildup of some negative charge (called a partial negative charge and designated δ−) on one side of the bond and some positive charge (designated δ+) on the other side of the bond. A covalent bond that has an unequal sharing of electrons, as in part (b) of Figure 4.5, is called a polar covalent bond. A covalent bond that has an equal sharing of electrons (part (a) of Figure 4.5) is called a nonpolar covalent bond.
Figure 4.5 Polar versus Nonpolar Covalent Bonds. (a) The electrons in the covalent bond are equally shared by both hydrogen atoms. This is a nonpolar covalent bond. (b) The fluorine atom attracts the electrons in the bond more than the hydrogen atom does, leading to an imbalance in the electron distribution. This is a polar covalent bond.
Any covalent bond between atoms of different elements is a polar bond, but the degree of polarity varies widely. Some bonds between different elements are only minimally polar, while others are strongly polar. Ionic bonds can be considered the ultimate in polarity, with electrons being transferred completely rather than shared. To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond.
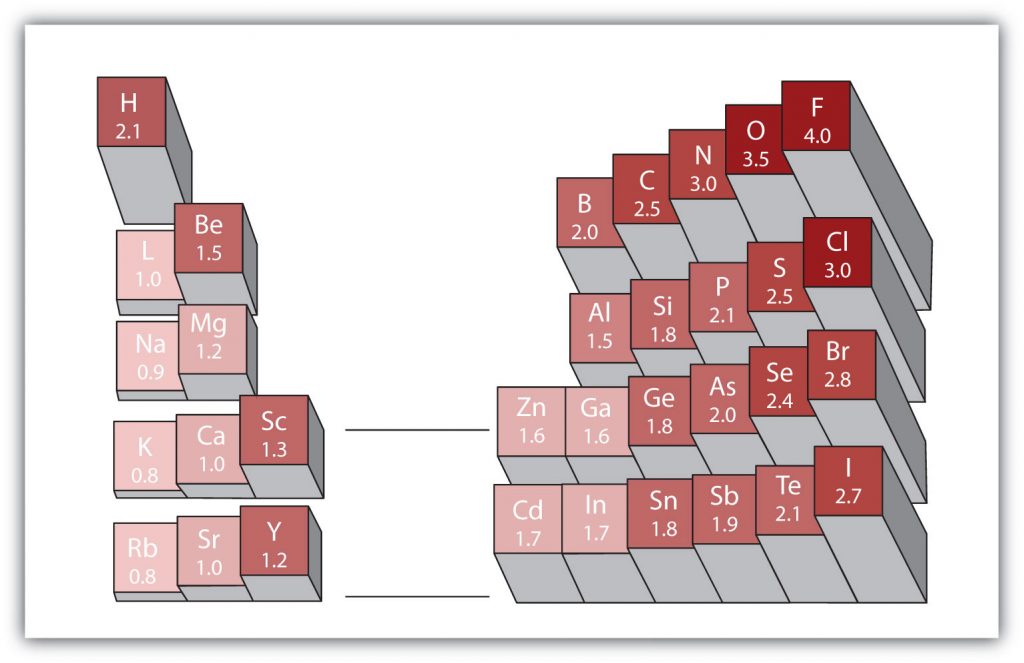
There are various numerical scales for rating electronegativity. Figure 4.6 shows one of the most popular—the Pauling scale. The polarity of a covalent bond can be judged by determining the difference in the electronegativities between the two atoms making the bond. The greater the difference in electronegativities, the greater the imbalance of electron sharing in the bond.
Figure 4.6 Electronegativities of Various Elements. The Pauling Scale for electronegativities has the value for fluorine atoms set at 4.0, the highest value.
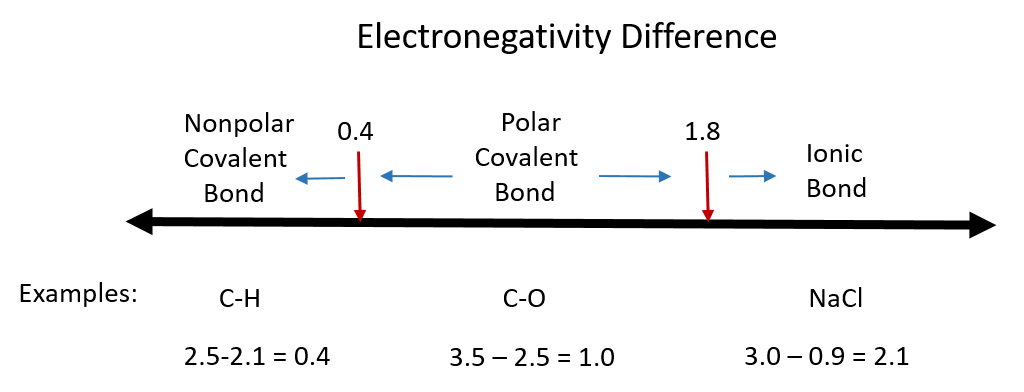
Although there are no hard and fast rules, the general rule is that a difference in electronegativity less than 0.4 indicates the bond is nonpolar; when the difference is greater than 0.4, the bond is considered polar. When the difference in electronegativities is large enough (generally greater than about 1.8), the resulting compound is considered ionic rather than covalent. An electronegativity difference of zero, of course, indicates a nonpolar covalent bond. Examples of electronegativity difference are shown in Figure 4.7.
Figure 4.7 Electronegativity Difference Diagram. The diagram above is a guide for discerning what type of bond forms between two different atoms. By taking the difference between the electronegativity values for each of the atoms involved in the bond, the bond type and polarity can be predicted. Note that full ionic character is rarely reached, however when metals and nonmetals form bonds, they are named using the rules for ionic bonding.
When a molecule’s bonds are polar, the molecule as a whole can display an uneven distribution of charge, depending on how the individual bonds are oriented. For example, the orientation of the two O–H bonds in a water molecule (Figure 4.8) is bent: one end of the molecule has a partial positive charge, and the other end has a partial negative charge. In short, the molecule itself is polar. The polarity of water has an enormous impact on its physical and chemical properties. (For example, the boiling point of water [100°C] is high for such a small molecule and is due to the fact that polar molecules attract each other strongly.) In contrast, while the two C=O bonds in carbon dioxide are polar, they lie directly opposite each other in the molecule and so cancel each other’s effects. Thus, carbon dioxide molecules are nonpolar overall. This lack of polarity influences some of carbon dioxide’s properties. (For example, carbon dioxide becomes a gas at −77°C, almost 200° lower than the temperature at which water boils.)
Figure 4.8 Physical Properties and Polarity. The physical properties of water (a) and carbon dioxide (b) are affected by their molecular polarities. Note that the arrows in the diagram always point in the direction where the electrons are more strongly attracted. In this diagram, the delta symbol (δ) is used with a (+) or (-) symbol to represent partial positive and partial negative charge distribution in polar covalent bonds. Note that the electrons shared in polar covalent bonds will be attracted to and spend more time around the atom with the higher electronegativity value. When the polarity is equal and directly opposing, as in the case of carbon dioxide (b), the overall molecule will have no overall charge.
4.4 Properties of Molecular Compounds
Molecular compounds have many properties that differ from ionic compounds. Some of the generalizations for this group include much lower melting and boiling points when compared with their ionic counterpoints. For example, water (H2O) has a melting point of 4oC and a boiling point of 100oC compared with NaCl that has a melting point of 801oC and a boiling point of 1,413oC. This is because the full charges created in ionic bonds have much stronger attractive force than the comparatively weak partial charges created in covalent molecules. thus, ionic compounds tend to form very strong crystalline lattice structures due to the repeating charges of the cation and anion components. Covalent compounds, on the otherhand, do not typically have such well-structured 3-dimensional shapes. Thus they tend to be more brittle and break more easily when in solid form, and many are found in liquid and gas phases. In addition, due to their lack of charges, they tend to be poor electrical and thermal conductors. Many are also insoluble in water due to their nonpolar nature (ie oil and water don’t mix).
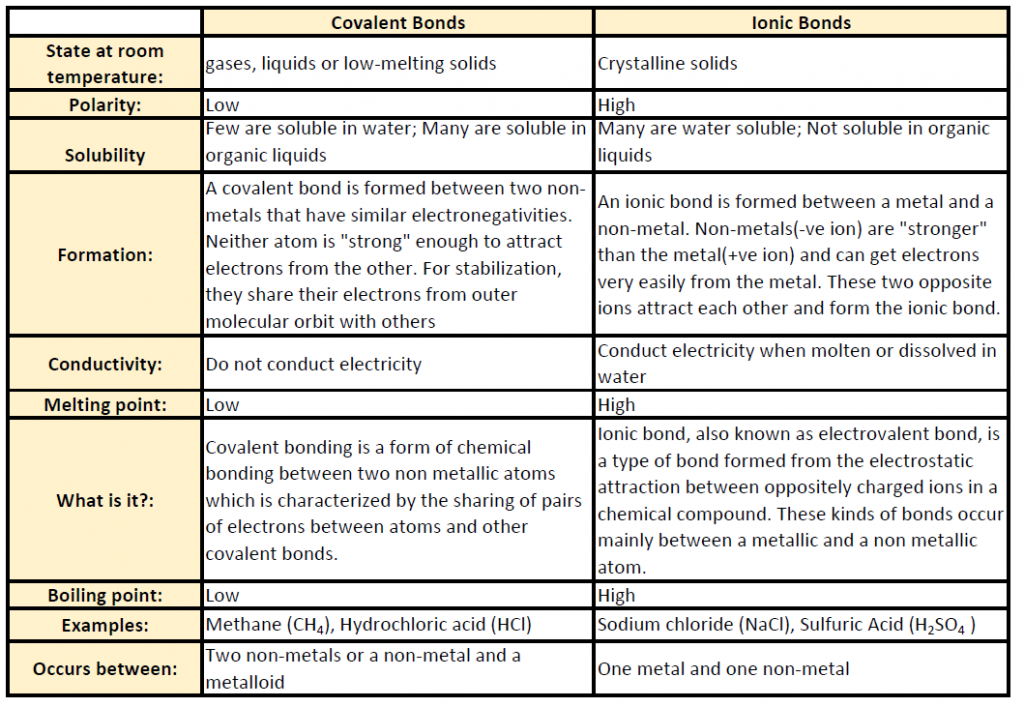
Table 4.1 shows common differences between covalent and ionic compounds.
Table 4.1 Comparison of Ionic and Covalent Compounds
4.5 Naming Binary Molecular Compounds
Recall that a molecular formula shows the number of atoms of each element that a molecule contains. A molecule of water contains two hydrogen atoms and one oxygen atom, so its formula is H2O. A molecule of octane, which is a component of gasoline, contains 8 atoms of carbon and 18 atoms of hydrogen. The molecular formula of octane is C8H18. When writing the chemical formula the element that is the least electronegative (the element that is farther left or further down within the same family group) is written first while the more electronegative element is written second. You will be required to know how to name simple binary covalent compounds (compounds composed of two different elements)
.

Figure 4.9 Nitrogen dioxide (NO2) is a reddish-brown toxic gas that is a prominent air pollutant produced by internal combustion engines.
The elements that combine to form binary molecular compounds are both nonmetal atoms or they are a combination of a nonmetal and a metalloid. This contrasts with ionic compounds, which were formed from a metal ion and a nonmetal ion. Therefore, binary molecular compounds are different because ionic charges cannot be used to name them or to write their formulas. Another difference is that two nonmetal atoms will frequently combine with one another in a variety of ratios. Consider the elements nitrogen and oxygen. They combine to make several compounds including
NO, NO2, and N2O
They all can’t be called nitrogen oxide. How would someone know which one you were talking about? Each of the three compounds has very different properties and reactivity. A system to distinguish between compounds such as these is necessary.
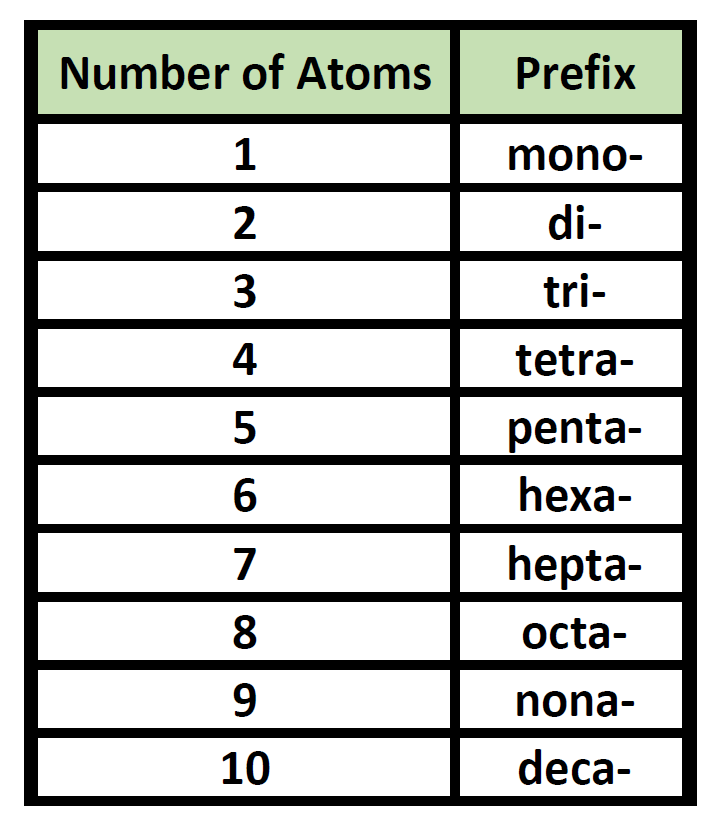
Prefixes are used in the names of binary molecular compounds to identify the number of atoms of each element. The table below shows the prefixes up to ten.
Table 4.2 Prefixes used for Nomenclature of Binary Covalent Molecules
The rules for using the prefix system of nomenclature of binary compounds can be summarized as follows.
- Generally, the less-electronegative element is written first in the formula, though there are a few exceptions. Exception 1: Carbon is always first in a formula. Exception 2: When hydrogen is participating in a covalent bond, it is typically written in the second postion (For example: hydrogen is after nitrogen in a formula such as NH3) Overall, the order of common nonmetals in binary molecular compounds is C, P, N, H, S, I, Br, Cl, O,
- When naming the first element, use the full name of the element and the appropriate prefix if there are more than one atom of that element in the formula. If there is only one atom for the first element, the term mono- is NOT used, but is implied. For example, CO is carbon monoxide, not monocarbon monoxide.
- For the second element the ending of the element’s name is typically changed to ‘-ide’ and the appropriate prefix is always used for the second element.
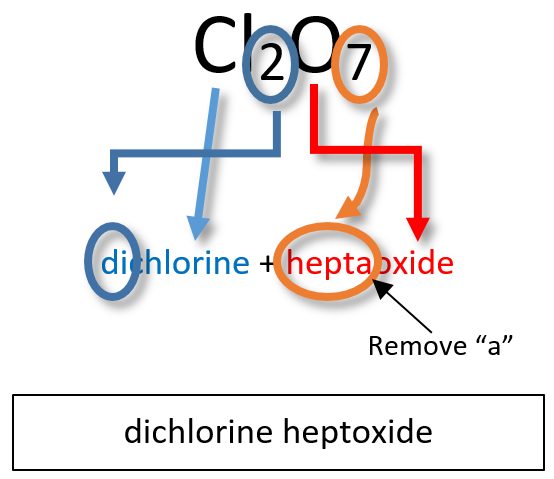
Note: the a or o at the end of a prefix is usually dropped from the name when the name of the element begins with a vowel. As an example, four oxygen atoms, is tetroxide instead of tetraoxide. Some examples of molecular compounds are listed in Table 4.3.
Table 4.3 Examples of Naming Covalent Molecules
Notice that the mono- prefix is not used with the nitrogen in the first compound, but is used with the oxygen in both of the first two examples. The S2Cl2 emphasizes that the formulas for molecular compounds are not reduced to their lowest ratios. The o of the mono- and the a of hepta- are dropped from the name when paired with oxide. For example:
4.6 Chapter Summary
Atoms can share pairs of valence electrons to obtain a valence shell octet. This sharing of electrons is a covalent bond. A species formed from covalently bonded atoms is a molecule and is represented by a molecular formula, which gives the number of atoms of each type in the molecule. The two electrons shared in a covalent bond are called a bonding pair of electrons. The electrons that do not participate in covalent bonds are called nonbonding pairs (or lone pairs) of electrons. A covalent bond consisting of one pair of shared electrons is called a single bond.
Covalent bonds occur between nonmetal atoms. Naming simple covalent compounds follows simple rules similar to those for ionic compounds. However, for covalent compounds, numerical prefixes are used as necessary to specify the number of atoms of each element in the compound.
In some cases, more than one pair of electrons is shared to satisfy the octet rule. Two pairs of electrons are shared by two atoms to make a double bond. Three pairs of atoms are shared to make a triple bond. Single, double, and triple covalent bonds may be represented by one, two, or three dashes, respectively, between the symbols of the atoms. In the case of a coordinate covalent bond, one atom supplies both of the electrons and the other atom does not supply any of the electrons.
To judge the relative polarity of a covalent bond, chemists use electronegativity, which is a relative measure of how strongly an atom attracts electrons when it forms a covalent bond. The greater the electronegativity difference between the atoms involved in the covalent bond, the more polarity the bond displays.
In comparison to ionic compounds, covalent molecules tend to have lower melting and boiling points, are less soluble in water, and are poor conductors of electricity. These major differences are largely due to increased polarity of ionic bonds when compared with covalent bonds.
4.7 References
Chapter 4 materials have been adapted from the following creative commons resources unless otherwise noted:
1. Organic Chemistry Portal. WikiUniversity. Available at: https://en.wikiversity.org/wiki/Portal:Organic_chemistry
2. Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html
3. Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
4. Molecules and Molecular Compounds. (2017) Libretexts. Available at: https://chem.libretexts.org/Textbook_Maps/General_Chemistry_Textbook_Maps/Map%3A_Chemistry%3A_The_Central_Science_(Brown_et_al.)/02._Atoms,_Molecules,_and_Ions/2.6%3A_Molecules_and_Molecular_Compounds
5. Clark, J. (2017) ‘General Principles of Chemical Bonding’ Published by Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Coordinate_(Dative_Covalent)_Bonding
6. OpenStax (2015) Atoms, Isotopes, Ions, and Molecules: The Building Blocks. OpenStax CNX.Available at: http://cnx.org/contents/be8818d0-2dba-4bf3-859a-737c25fb2c99@12.
7. Wikipedia, Ionic Compound. Available at: https://en.wikipedia.org/wiki/Ionic_compound
8. Physical and Theoretical Chemistry (2017) Libretexts. Available at: https://chem.libretexts.org/Core/Physical_and_Theoretical_Chemistry/Chemical_Bonding/General_Principles_of_Chemical_Bonding/Covalent_Bonds_vs_Ionic_Bonds.