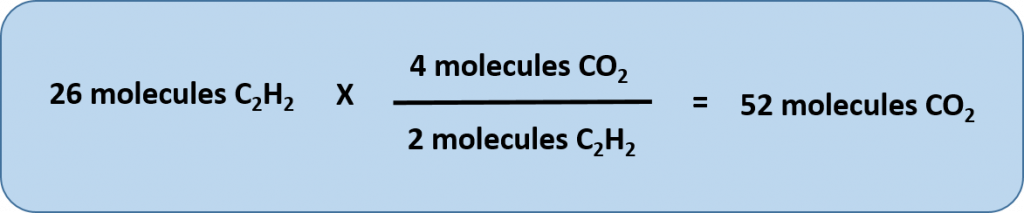Home » Student Resources » Online Chemistry Textbooks » CH150: Preparatory Chemistry » CH150: Chapter 5 – Chemical Reactions
MenuCH150: Chapter 5 – Chemical Reactions
Chapter 5: Chemical Reactions
This content can also be downloaded as an printable PDF, adobe reader is required for full functionality.
This text is published under creative commons licensing, for referencing and adaptation, please click here.
Opening Essay
5.1 The Law of Conservation of Matter
5.2 Writing and Balancing Chemical Equations
Practice Writing and Balancing Equations
5.3 Quantitative Relationships Based on Chemical Equations
5.4 Some Types of Chemical Reactions
(1) Combination (or Synthesis) Reactions
(2) Decomposition Reactions
(3) Single Replacement (or Single Displacement) Reactions
(4) Double Replacement (or Displacement) Reactions
Acid-Base Neutralization Reactions
Precipitation Reactions
Solubility Rules
Net Ionic Equations
Applications and Examples
(5) Oxidation and Reduction (Redox) Reactions
Rules for Assigning Oxidation States
Combustion Reactions
A Closer Look at the Importance of Redox Reactions
5.5 Chapter Summary
5.6 References
Opening Essay
Although yeast has been used for thousands of years, its true nature has been known only for the last two centuries. Yeasts are single-celled fungi. About 1,000 species are recognized, but the most common species is Saccharomyces cerevisiae, which is used in bread making. Other species are used for the fermentation of alcoholic beverages. Some species can cause infections in humans.
Yeasts live primarily on sugars, such as glucose (C6H12O6). They convert glucose into carbon dioxide (CO2) and ethanol (C2H5OH) in a chemical transformation that is represented as follows:
C6H12O6 (s) → 2CO2(g) + 2C2H5OH(ℓ)
Bread making depends on the production of carbon dioxide. The gas, which is produced in tiny pockets in bread dough, acts as a leavening agent: it expands during baking and makes the bread rise. Leavened bread is softer, lighter, and easier to eat and chew than unleavened bread. The other major use of yeast, fermentation, depends on the production of ethanol, which results from the same chemical transformation. Some alcoholic beverages, such as champagne, can also be carbonated using the carbon dioxide produced by the yeast.
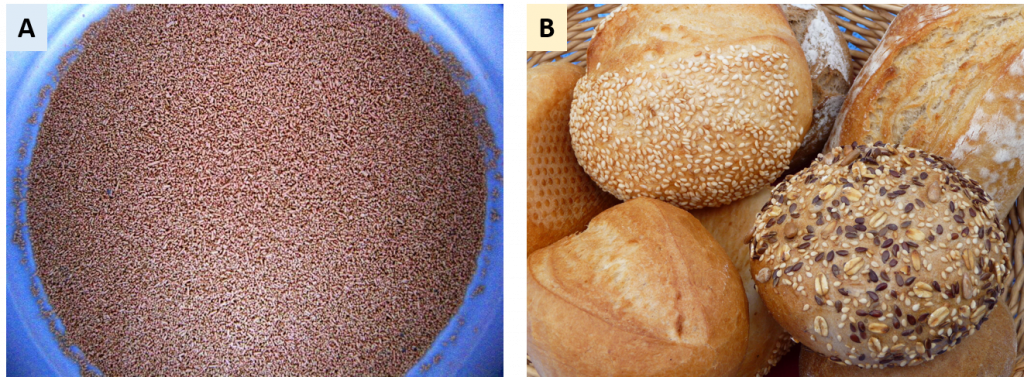
Figure 5.1 Yeast is used in many food processing applications. In bread making, the yeast, Saccharomyces cerevisiae, is often sold as a granulated dry form (A). It is used to create leavened bread (B).
Photo of activated dry yeast is from: Ranveig. Photo of leavened bread is from: 3268zauber
Yeast is among the simplest life forms on Earth, yet it is absolutely necessary for at least two major food industries. Without yeast to turn dough into bread and juice into wine, these foods and food industries would not exist today.
5.1 The Law of Conservation of Matter
Chemical change is a central concept in chemistry. The goal of chemists is to know how and why a substance changes in the presence of another substance or even by itself. Because there are tens of millions of known substances, there are a huge number of possible chemical reactions. In this chapter, we will find that many of these reactions can be classified into a small number of categories according to certain shared characteristics.
In science, a law is a general statement that explains a large number of observations. Before being accepted, a law must be verified many times under many conditions. Laws are therefore considered the highest form of scientific knowledge and are generally thought to be inviolable. Scientific laws form the core of scientific knowledge.
One scientific law that provides the foundation for understanding in chemistry is the law of conservation of matter. It states that in any given system that is closed to the transfer of matter (in and out), the amount of matter in the system stays constant. A concise way of expressing this law is to say that the amount of matter in a system is conserved, or that matter cannot be created or destroyed during a chemical reaction. It only changes form.
What does this mean for chemistry? In any chemical change, one or more initial substances change into a different substance or substances. Both the initial and final substances are composed of atoms because all matter is composed of atoms. According to the law of conservation of matter, matter is neither created nor destroyed, so we must have the same number and type of atoms after the chemical change as were present before the chemical change.
Before looking at explicit examples of the law of conservation of matter, we need to examine the method chemists use to represent chemical changes.
5.2 Writing and Balancing Chemical Equations
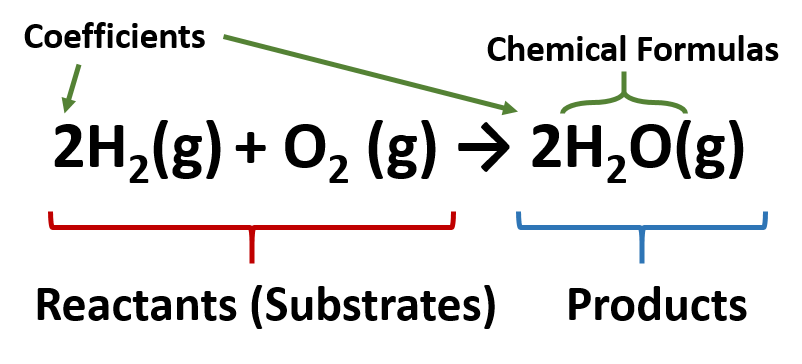
Water (H2O) is composed of hydrogen and oxygen. Suppose we imagine a process in which we take some elemental hydrogen (H2) and elemental oxygen (O2) and let them react to make water. The chemical equation below is used to express this reaction:
In this chemical reaction, the chemical formulas of the reactants (or substrates) are written on the left side of the equation, and the chemical formulas of the products are written on the right side. A plus sign connects the initial substances (and final substances, if there is more than one), and an arrow (→) represents the chemical change (or reaction). In reactions, it is also common to include a phase label with each formula—(s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution.
Due to the law of the conservation of matter, each chemical reaction needs to be balanced to ensure that the same number and types of atoms on the left side of the equation are the same as on the right side of the reaction. To represent this balance, coefficients are placed in front of the chemical formulas on each side to ensure that the same numbers and types of atoms are on each side of the equation. If the coefficient is one, it is not written into the equation, but is assumed. The coefficients represent how many molecules are present in the reaction. In this case, we can see that there are two H2 molecules (or a total of four hydrogen atoms) of the left side of the equation and that there are two H2O molecules, which also include four hydrogens on the right side of the equation. Likewise, there are two oxygen atoms in the form of one oxygen molecule (O2) on the left side, and two oxygen atoms on the right side in the form or two water molecules. A diagram using the Lewis structures is shown below to represent the number of molecules indicated by the coefficients of the equation:
Figure 5.2 shows a rather dramatic example of this very reaction.
Figure 5.2 Chemical reactions can be violent in nature. When exposed to a spark or a flame, hydrogen and oxygen react violently to form water. Here the hydrogen gas in the zeppelin, SS Hindenburg, reacts with the oxygen in the air to make water.
The Hindenburg Photo is made available from the US Navy.
Practice Writing and Balancing Equations
To write proper chemical reactions, it is first necessary to write the correct chemical formulas. As you have learned in Chapters 3 and 4, both ionic and covalent compounds combine to either donate/accept electrons or share electrons in ways that enable atoms to reach a stable state, most often following the octet rule. This should be the first consideration when writing a chemical reaction.The second consideration should be the states of the matter involved in the chemical reaction. Label each as a solid (s), a liquid (ℓ), a gas (g), or an aqueous solution (aq). Once the correct chemical formulas have been established, proper chemical equations should then be balanced. Writing balanced reactions is a chemist’s way of acknowledging the law of conservation of matter.
How does one balance a chemical equation, starting with the correct formulas of the reactants and products? Basically, a back-and-forth approach is adopted, counting the number of atoms of one element on one side, checking the number of atoms of that element on the other side, and changing a coefficient if necessary. Then check another element, going back and forth from one side of the equation to another, until each element has the same number of atoms on both sides of the arrow. In many cases, it does not matter which element is balanced first and which is balanced last, as long as all elements have the same number of atoms on each side of the equation. However, if elemental forms appear in an equation (i.e. Na, Zn, O2, H2, Cl2, etc), it is often easier to balance the equation, by leaving the elemental forms to be balanced last.
For example, to balance the equation:
CH4 + Cl2 → CCl4 + HCl
the first thing that we notice, is that it contains the elemental form of chlorine (Cl2). That leaves carbon and hydrogen to balance. We might choose to count the carbon atoms first, finding that both sides are balanced with one carbon atom. Thus, we will not change the coefficient for the carbon-containing molecules, but instead shift our focus to the number of hydrogen atoms. The reactant side has four hydrogen atoms, however, the product side only has one hydrogen. Thus, we will need to add a coefficient to the product side, such that the product side will also have four hydrogen atoms. We fix this by putting a 4 in front of the HCl:
CH4 + Cl2 → CCl4 + 4HCl
Now each side has four hydrogen atoms. Now we can balance the chlorine. The product side has a total of eight chlorine atoms (four from the CCl4 and four from the four molecules of HCl), so we need eight chlorine atoms on the reactant-side of the equation. Because elemental chlorine is a diatomic molecule, we need a total of four chlorine molecules to get a total of eight chlorine atoms. We add another 4 in front of the Cl2 reactant:
CH4 + 4Cl2 → CCl4 + 4HCl
Now we double check: each side has one carbon atom, four hydrogen atoms, and eight chlorine atoms. Yes, the chemical equation is balanced.
Quiz Yourself: More Practice Balancing Equations
5.3 Quantitative Relationships Based on Chemical Equations
A balanced chemical equation not only describes some of the chemical properties of substances—by showing us what substances react with what other substances to make what products—but also shows numerical relationships between the reactants and the products. The study of these numerical relationships is called stoichiometry. The stoichiometry of chemical equations revolves around the coefficients in the balanced chemical equation because these coefficients determine the molecular ratio in which reactants react and products are made. It is very similar to cooking. For example, to make a hamburger, for each hamburger patti, you need to have two slices of bread for the bun. Thus, the ratio of bread to hamburger is 2 to 1.
Consider the following balanced chemical equation:
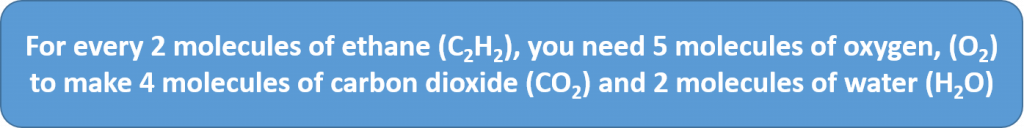
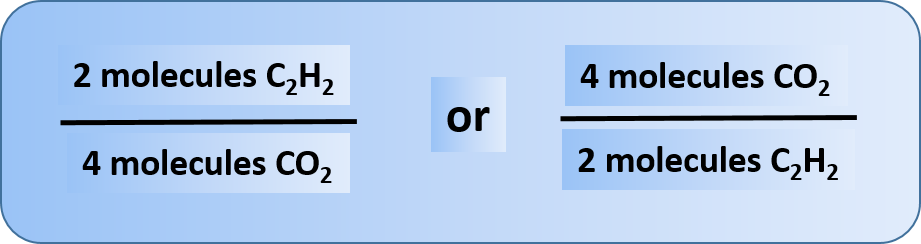
2C2H2 + 5O2 → 4CO2 + 2H2O
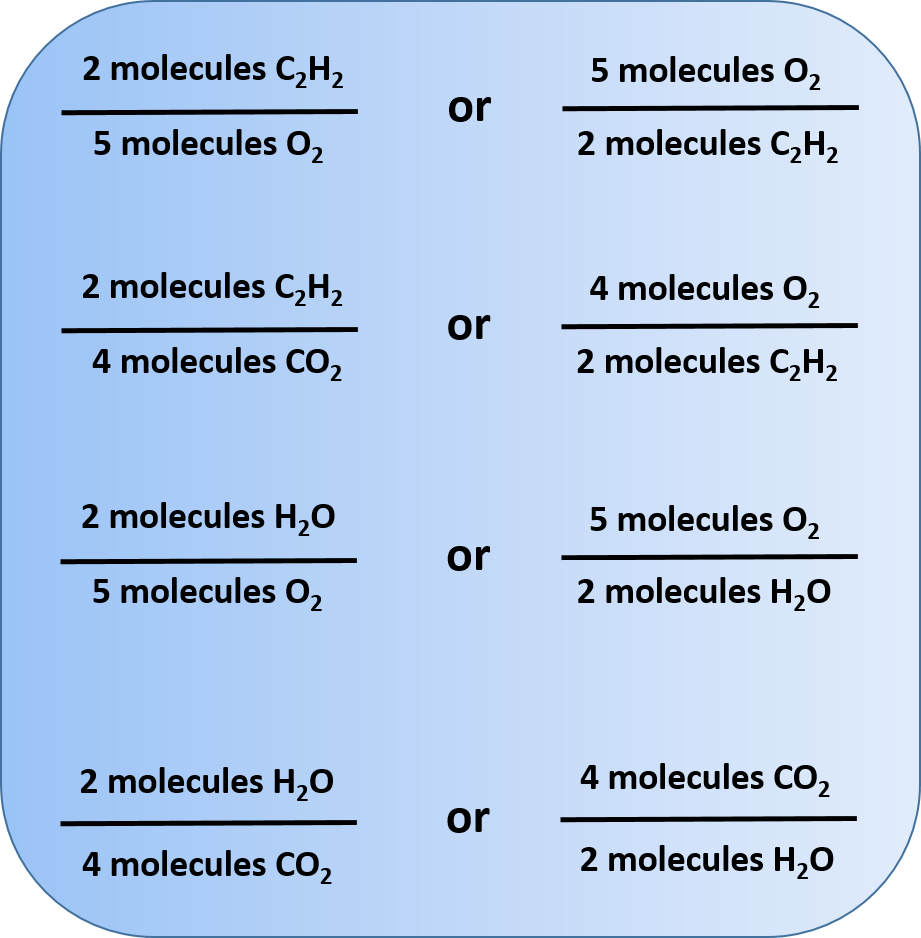
The coefficients on the chemical formulas give the ratios in which the reactants combine and the products form. Thus, we can make the following statements and construct the following ratios:
STATEMENT:
This statement can then be represented mathematically into ratios that represent these written relationship statements.
Other relationships are possible; in fact, 12 different ratios can be constructed from this balanced chemical equation. In each ratio, note that the unit is molecules. Chemical reactions describe the number of molecules of one substance required to react with or produce another substance.
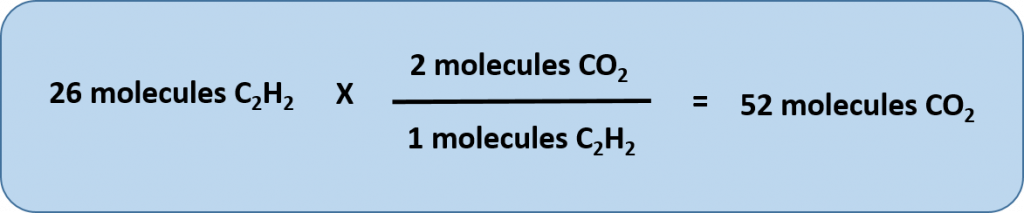
Any of these fractions can be used as a conversion factor to relate an amount of one substance to an amount of another substance. For example, suppose we want to know how many CO2 molecules are formed when 26 molecules of C2H2 are reacted.
As usual with a conversion problem, we start with the amount we are given — 26 C2H2 — and multiply it by a conversion factor that cancels out our original unit and introduces the unit we are converting to—in this case, CO2.
Thus, we have two choices for our conversion factor
To cancel C2H2, we need to make sure that this unit is on the bottom of our convesion factor. Thus, we can set up our conversion equation as follows:
Thus, 52 molecules of CO2 are formed when we start with 26 molecules of C2H2. Note that with this conversion factor, that we could have simplified our conversion factor prior to completing the multiplication and division steps, as follows:
This application of stoichiometry is extremely powerful in its predictive ability, as long as we begin with a balanced chemical equation. Without a balanced chemical equation, the predictions made by simple stoichiometric calculations will be incorrect.
5.4 Some Types of Chemical Reactions
Although there are untold millions of possible chemical reactions, most can be classified into a small number of general reaction types. Classifying reactions has two purposes: it helps us to recognize similarities among them, and it enables us to predict the products of certain reactions. In this chapter, we will discuss five major categories of chemical reactions: (1) Combination (or Synthesis) Reactions, (2) Decomposition Reactions, (3) Single Replacement Reactions, (4) Double Replacement Reactions, and (5) Redox Reactions. Note that a particular reaction may fall into more than one of the categories that we will define in this book.
(1) Combination (or Synthesis) Reactions
A combination (or synthesis) reaction is a chemical reaction is a building reaction, where two or more elements or compounds combine to make a single compound.
The general equation for a combination reaction is:
A + B → AB
An example of a combination reaction is:
4Fe(s) + 3O2(g) → 2Fe2O3(s)
In this reaction, the elements iron (Fe) and oxygen (O2) combine to produce iron III oxide (Fe2O3). Note that combination reactions do not have to combine elements together, however. Combination reactions can also combine compounds together to form larger compounds. The chemical equation:
Fe2O3(s) + 3SO3(s) → Fe2(SO4)3(s)
shows a combination reaction in which iron III oxide (Fe2O3) combines with three molecules of sulfur trioxide (SO3) to make iron III sulfate [Fe2(SO4)3].
(2) Decomposition Reactions
A decomposition reaction is the reverse of a combination reaction. In a decomposition reaction, a single substance is converted or broken down into two or more products. A general equation for decomposition reactions is:
AB → A + B
For example, the equation below is a decomposition reaction that occurs when Sodium Bicarbonate (NaHCO3) is exposed to heat:
2NaHCO3(s) → Na2CO3(s) + CO2(g) + H2O(ℓ)
Another example is the decomposition of KClO3:
2KClO3(s) → 2KCl(s) + 3O2(g)
This reaction can be used to generate small amounts of oxygen in the chemistry lab.
(3) Single Replacement (or Single Displacement) Reactions
Single replacement (or displacement) reactions occur when an element (most frequently a metal) replaces another element from a compound. They can occur in two forms: cationic exchange or anionic exchange. In cationic exchanges, the positive ions (cations) of the equation change place. In anionic exchanges, the negative ions (anions) change place. Note that single replacement reactions are often also redox reactions, and thus, we will revisit this reaction type in that section.
The general equation for a cationic single replacement reaction is:
A + BC → AC + B
For a cationic exchange reaction, A represents a cation that replaces cation B in the product, AC. An example of a cationic exchange reaction is:
2 Ag(s) + 2 HCl(aq) → H2(g) + 2 AgCl(s)
In this reaction, the silver replaces the hydrogen cation in the hydrochloric acid to form silver chloride in the final product.
For anion exchange reactions, the following general reaction can be used:
D + BC → BD + C
An example of an anionic exchange reaction is:
3 F2(g) + 2 FeCl3(aq) → 2 FeF3(aq) + 3 Cl2(g)
In this reaction, flourine replaces chlorine anion in the iron III chloride to produce iron III fluoride.
(4) Double Replacement (or Displacement) Reactions
Double replacement (or displacement) reactions occur when two chemical groups substitute for each other (change partners) in their respective compounds.
A general equation for a double replacement reaction is:
AB + CD → AD + CB
Two major types of double replacement reactions are Acid-Base Neutralization reactions and Precipitation reactions.
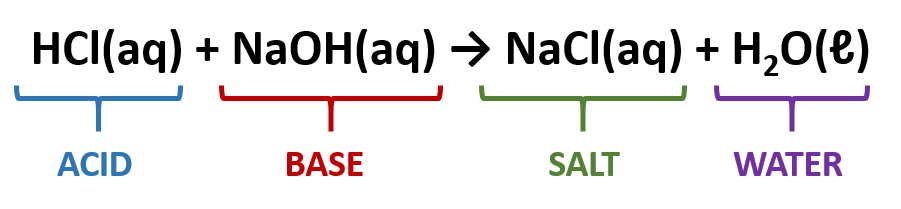
Acid-Base Neutralization Reactions
In an acid base neutralization reaction, an acid is combined with a base to form a salt and water.
Precipitation Reactions
Precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common ionic solids. Because not all aqueous reactions form precipitates, one must consult the solubility rules before determining the state of the products The ability to predict these reactions allows scientists to determine which ions are present in a solution, and allows industries to form chemicals by extracting components from these reactions.
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary. Some reactions depend on temperature, such as solutions used for buffers, whereas others are dependent only on solution concentration. The solids produced in precipitate reactions are crystalline solids, and can be suspended throughout the liquid or fall to the bottom of the solution. The remaining fluid is called supernatant liquid. The two components of the mixture (precipitate and supernate) can be separated by various methods, such as filtration, centrifuging, or decanting.
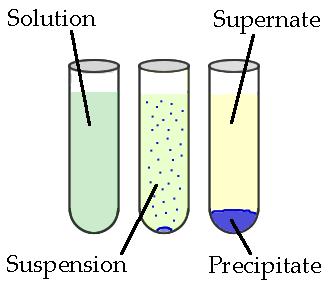
Figure 5.3: Above is a diagram of the formation of a precipitate in solution.
The use of solubility rules require an understanding of the way that ions react. Most precipitation reactions are single replacement reactions or double replacement reactions. A double replacement reaction occurs when two ionic reactants dissociate and bond with the respective anion or cation from the other reactant. The ions replace each other based on their charges as either a cation or an anion. This can be thought of as “switching partners”; that is, the two reactants each “lose” their partner and form a bond with a different partner:

Figure 5.4: A double replacement reaction
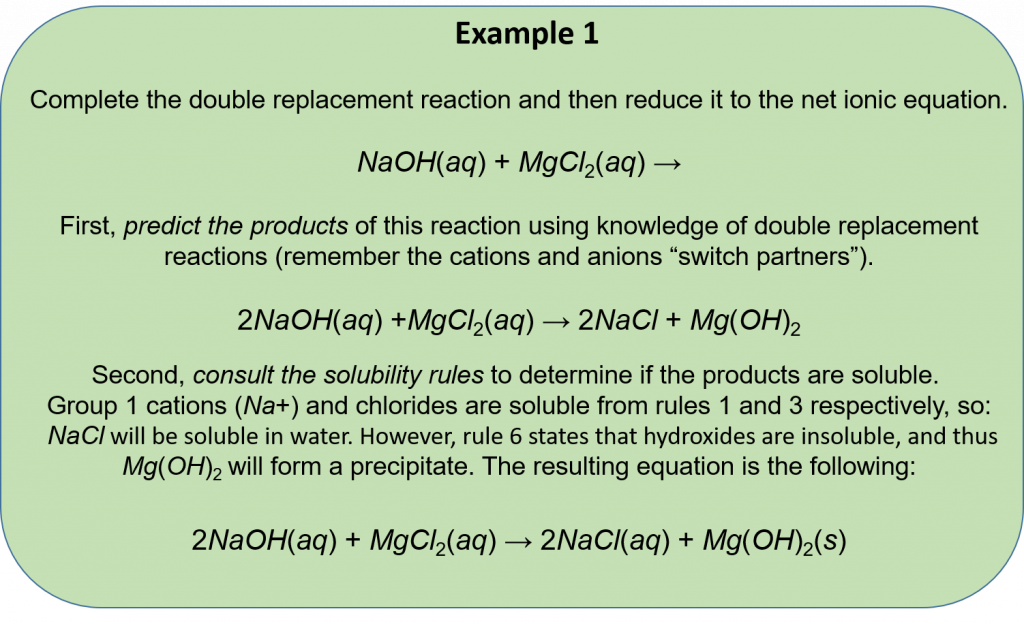
A double replacement reaction is specifically classified as a precipitation reaction when the chemical equation in question occurs in aqueous solution and one of the of the products formed is insoluble and forms a solid product. An example of a precipitation reaction is given below:
CdSO4(aq) + K2S(aq) → CdS(s) + K2SO4(aq)
Both reactants are aqueous and one product is solid. Because the reactants are ionic and aqueous, they dissociate and are therefore soluble. However, CdS is insoluble and precipitate out of solution to form a solid product. There are six solubility guidelines that you can use to help you predict which molecules will form a solid and enable you to identify a precipitation reaction.
Solubility Rules
Whether or not a reaction forms a precipitate is dictated by the solubility rules. These rules provide guidelines that tell which ions form solids and which remain in their ionic form in aqueous solution. The rules are to be followed from the top down, meaning that if something is insoluble (or soluble) due to rule 1, it has precedence over a higher-numbered rule.
- Salts formed with group 1 cations and NH4+ cations are soluble. There are some exceptions for certain Li+ salts.
- Acetates (C2H3), nitrates (NO3−), and perchlorates (ClO4−) are soluble.
- Bromides, chlorides, and iodides are soluble.
- Sulfates (SO42-) are soluble with the exception of sulfates formed with Ca2+, Sr2+, and Ba2+. These salts are insoluble.
- Salts containing silver, lead, and mercury (I) are insoluble.
- Carbonates (CO32−), phosphates (PO43−), sulfides, oxides, and hydroxides (OH−) are insoluble. Sulfides formed with group 2 cations and hydroxides formed with calcium, strontium, and barium are exceptions.
If the rules state that an ion is soluble, then it remains in its aqueous ion form. If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from the other reactant. If all the ions in a reaction are shown to be soluble, then no precipitation reaction occurs.
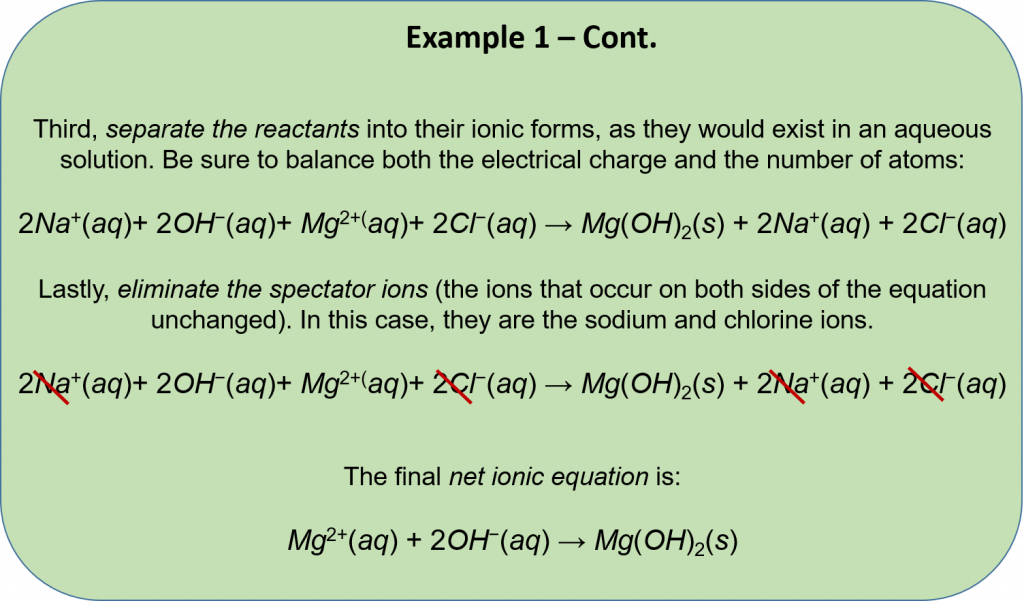
Net Ionic Equations
To understand the definition of a net ionic equation, recall the equation for the double replacement reaction. Because this particular reaction is a precipitation reaction, states of matter can be assigned to each variable pair:
AB(aq) + CD(aq) → AD(aq) + CB(s)
The first step to writing a net ionic equation is to separate the soluble (aqueous) reactants and products into their respective cations and anions. Precipitates do not dissociate in water, so the solid should not be separated. The resulting equation looks like that below:
A+(aq) + B–(aq) + C+(aq) + D–(aq) → A+(aq) + D–(aq) + CB(s)
In the equation above, A+and D– ions are present on both sides of the equation. These are called spectator ions because they remain unchanged throughout the reaction. Since they go through the equation unchanged, they can be eliminated to show the net ionic equation:
C+ (aq)+ B– (aq) → CB (s)
The net ionic equation only shows the precipitation reaction. A net ionic equation must be balanced on both sides not only in terms of atoms of elements, but also in terms of electric charge. Precipitation reactions are usually represented solely by net ionic equations. If all products are aqueous, a net ionic equation cannot be written because all ions are canceled out as spectator ions. Therefore, no precipitation reaction occurs.
Applications and Examples
Precipitation reactions are useful in determining whether a certain element is present in a solution. If a precipitate is formed when a chemical reacts with lead, for example, the presence of lead in water sources could be tested by adding the chemical and monitoring for precipitate formation. In addition, precipitation reactions can be used to extract elements, such as magnesium from seawater. Precipitation reactions even occur in the human body between antibodies and antigens; however, the environment in which this occurs is still being studied.
Practice Problems
Write the net ionic equation for the potentially double displacement reactions. Make sure to include the states of matter and balance the equations.
- Fe(NO3)3(aq) + NaOH(aq) →
- Al2(SO4)3(aq) + BaCl2(aq) →
- HI(aq) + Zn(NO3)2(aq) →
- CaCl2(aq) + Na3PO4(aq) →
- Pb(NO3)2(aq) + K2SO4(aq)
Solutions
1. Regardless of physical state, the products of this reaction are Fe(OH)3 and NaNO3. The solubility rules predict that NaNO3 is soluble because all nitrates are soluble (rule 2). However, Fe(OH)3 is insoluble, because hydroxides are insoluble (rule 6) and Fe is not one of the cations which results in an exception. After dissociation, the ionic equation is as follows:
Canceling out spectator ions leaves the net ionic equation:
2. From the double replacement reaction, the products are AlCl3 and BaSO4. AlCl3 is soluble because it contains a chloride (rule 3); however, BaSO4 is insoluble: it contains a sulfate, but the Ba2+ ion causes it to be insoluble because it is one of the cations that causes an exception to rule 4. The ionic equation is (after balancing):
Canceling out spectator ions leaves the following net ionic equation:
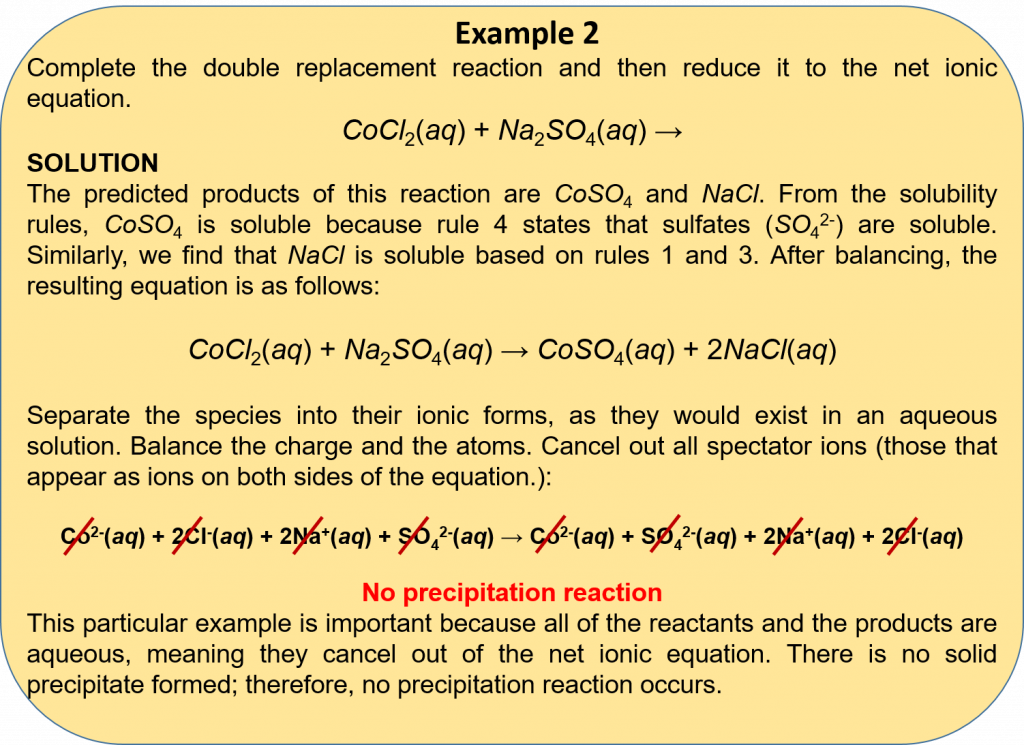
3. From the double replacement reaction, the products HNO3 and ZnI2 are formed. Looking at the solubility rules, HNO3 is soluble because it contains nitrate (rule 2), and ZnI2 is soluble because iodides are soluble (rule 3). This means that both the products are aqueous (i.e. dissociate in water), and thus no precipitation reaction occurs.
4. The products of this double replacement reaction are Ca3(PO4)2 and NaCl. Rule 1 states that NaCl is soluble, and according to solubility rule 6, Ca3(PO4)2 is insoluble. The ionic equation is:
After canceling out spectator ions, the net ionic equation is given below:
5. The first product of this reaction, PbSO4, is soluble according to rule 4 because it is a sulfate. The second product, KNO3, is also soluble because it contains nitrate (rule 2). Therefore, no precipitation reaction occurs.
(5) Oxidation and Reduction (Redox) Reactions
An oxidation-reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two atoms or compounds. The substance that loses the electrons is said to be oxidized, while the substance that gains the electrons is said to be reduced. The change in electron composition can be evaluated in the change of the oxidation state (or number) of an atom. Therefore, an oxidation-reduction reaction is any chemical reaction in which the oxidation state (number) of a molecule, atom, or ion changes by gaining or losing an electron. We will learn how to evaluate the oxidation state of a molecule within this section. Overall, redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting.
As shown in figure XX, an easy mnemonic for helping you remember which member gains electrons and which member loses electrons is ‘LEO the lion says GER’, where LEO stands for Lose Electrons = Oxidized and GER stands for Gain Electrons = Reduced.
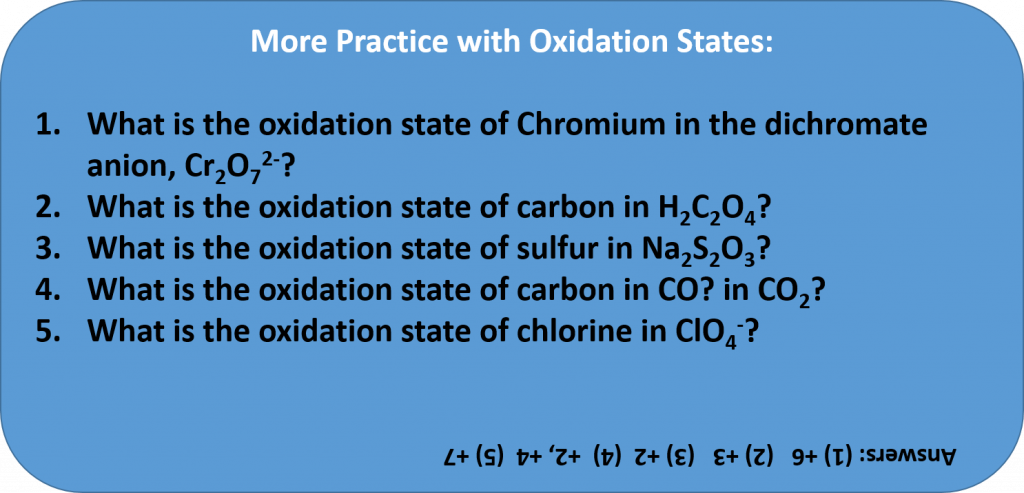
Rules for Assigning Oxidation States
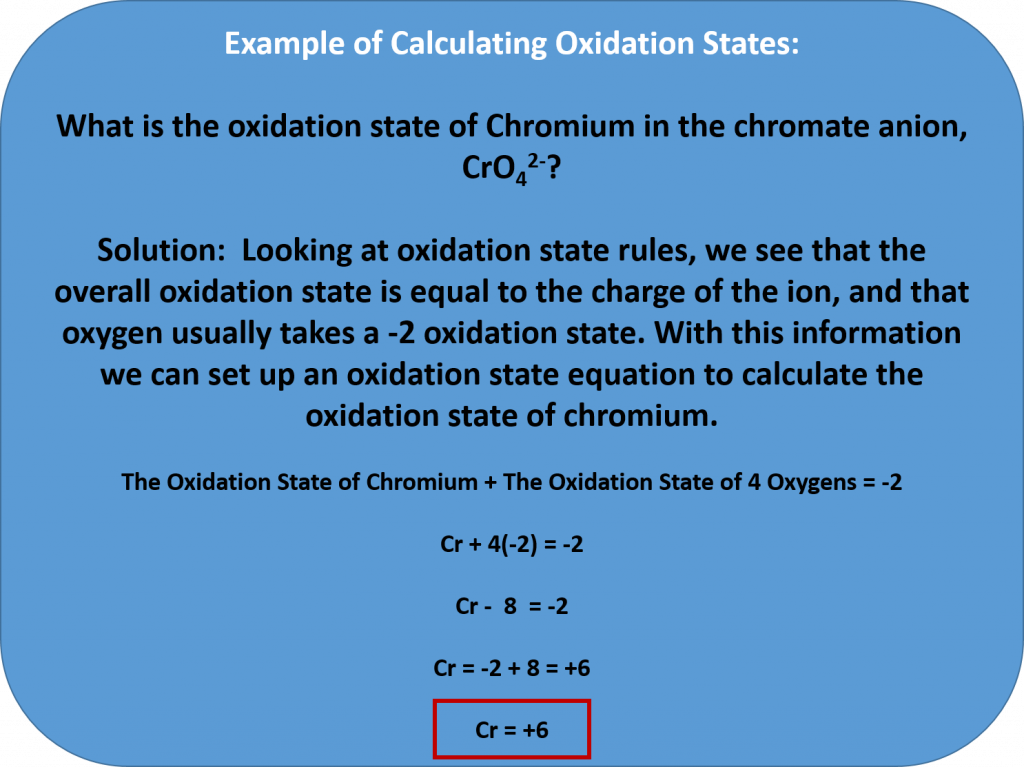
The oxidation state of an element corresponds to the number of electrons, e–, that an atom loses or gains during an ionic bond, or appears to lose/grain when joining in covalent bonds with other atoms in compounds. In determining the oxidation state of an atom, there are seven guidelines to follow:
- The oxidation state of an atom in its elemental form is 0. (This includes elemental forms that occur as diatomic molecules. For example, each oxygen in a molecule of O2, has an oxidation state = 0.)
- The total oxidation state of all atoms in a neutral species is 0 and in an ion is equal to the ion charge. (For example, the overall oxidation state of NaCl = 0, even though the oxidation state of Na+ in this bond is +1 and the oxidation state of the chlorine atom, Cl–, is -1. When you add them together they equal 0. In the case of an ion, the overall charge is always indicated. For example, the overall charge of a OH– ion is -1, while the oxygen in the OH– has a -2 oxidation state and the hydrogen has a +1 oxidation state.)
- Group 1 metals have an oxidation state of +1 and Group 2 an oxidation state of +2 when they are involved in ionic bonding.
- The oxidation state of fluorine is -1 in compounds
- Hydrogen generally has an oxidation state of +1 in compounds
- Oxygen generally has an oxidation state of -2 in compounds
- In binary metal compounds, Group 17 (or 7A) elements have an oxidation state of -1, Group 16 (or 6A) of -2, and Group 15 (or 5A) of -3.
- The oxidation states of other atoms are calculated based on rules 1-7.
Once you have mastered calculating oxidation states, you can start evaluating chemical reactions to determine if they are oxidation-reduction reactions.
Redox reactions are comprised of two parts, a reduced half and an oxidized half, that always occur together. The reduced half gains electrons and the oxidation number decreases (or becomes less positive), while the oxidized half loses electrons and the oxidation number increases (becomes more positive). There is no net change in the number of electrons in a redox reaction. Those given off in the oxidation half reaction are taken up by another species in the reduction half reaction.
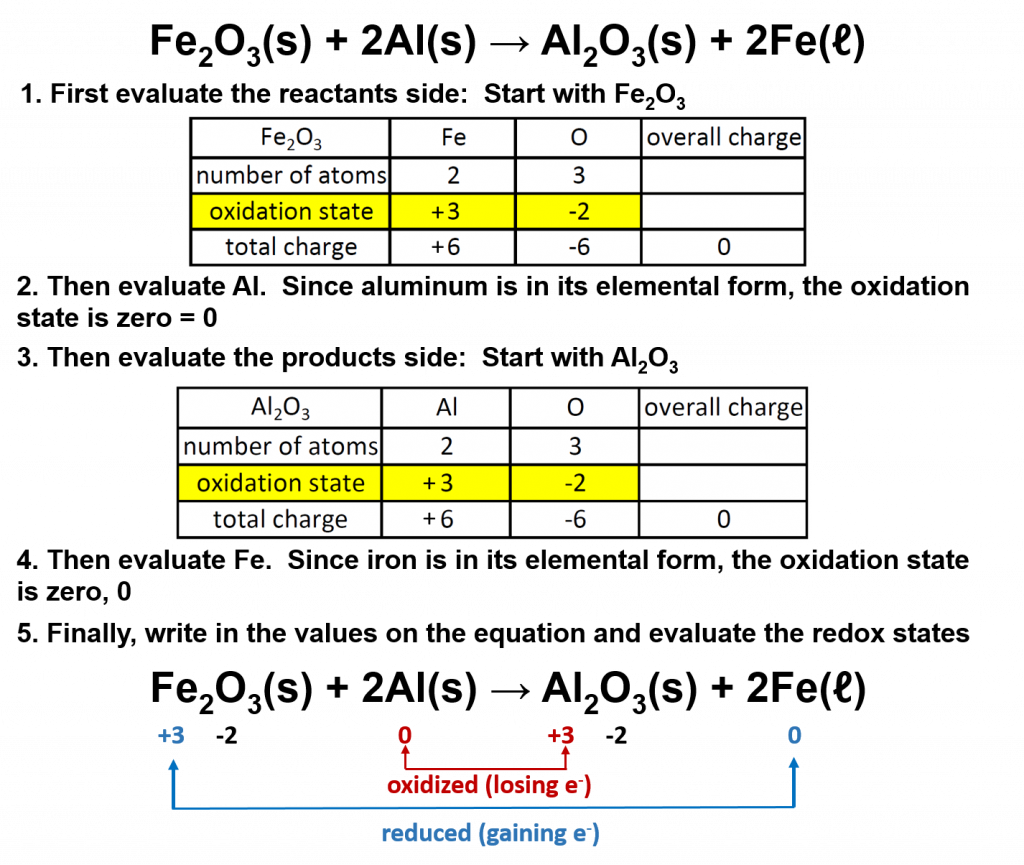
A good example of a redox reaction is the thermite reaction, in which iron atoms in ferric oxide lose (or give up) O atoms to Al atoms, producing Al2O3.
To recognize redox reactions, begin by determining the oxidation states of each atom in the reaction on both the left and the right sides of the equation. Then determine of the oxidation state of any of the atoms changes from the reactant to the product side. If so, then the reaction is a redox reaction, and you can determine which compound is oxidized (loses electrons) and which is reduced (gains electrons). For example, if we evaluate the thermite reaction above:
Overall in this reaction, the aluminum is oxidized to aluminum oxide, and the iron in the iron III oxide is reduced to elemental iron. Notice that there is no change to the oxidation state of oxygen.
The two species that exchange electrons in a redox reaction are given special names. The ion or molecule that accepts electrons (or becomes reduced during the reaction) is called the oxidizing agent; by accepting electrons it causes the oxidation of another compound or element. In the thermite reaction above, the iron III oxide is the oxidizing agent. Conversely, the species that donates electrons is called the reducing agent; when the reaction occurs, it reduces the other species. Thus, in the thermite reaction, the aluminum is the reducing agent. In other words, the substance that is oxidized is the reducing agent and the substance that is reduced is the oxidizing agent.
Single replacement reactions are often also redox reactions. A good indicator of a redox reaction is the appearance of an elemental form of a substance on one side that is converted into a compound on the other side. Another common redox reaction is a combustion reaction.
Combustion Reactions
Combustion reactions almost always involve oxygen in the form of O2, and are almost always exothermic, meaning they produce heat. Chemical reactions that give off light and heat and light are colloquially referred to as “burning.” Complete combustion of carbon compounds results in the production of carbon dioxide (CO2) and water (H2O). Note that carbon can exist in a range of oxidation states, typically from -4 to +4. The burning of fuels that provides the energy to maintain our civilization and the metabolism of foods that furnish the energy that keeps us alive both involve redox reactions.
All combustion reactions are also redox reactions.
CxHy + O2 → CO2 + H2O
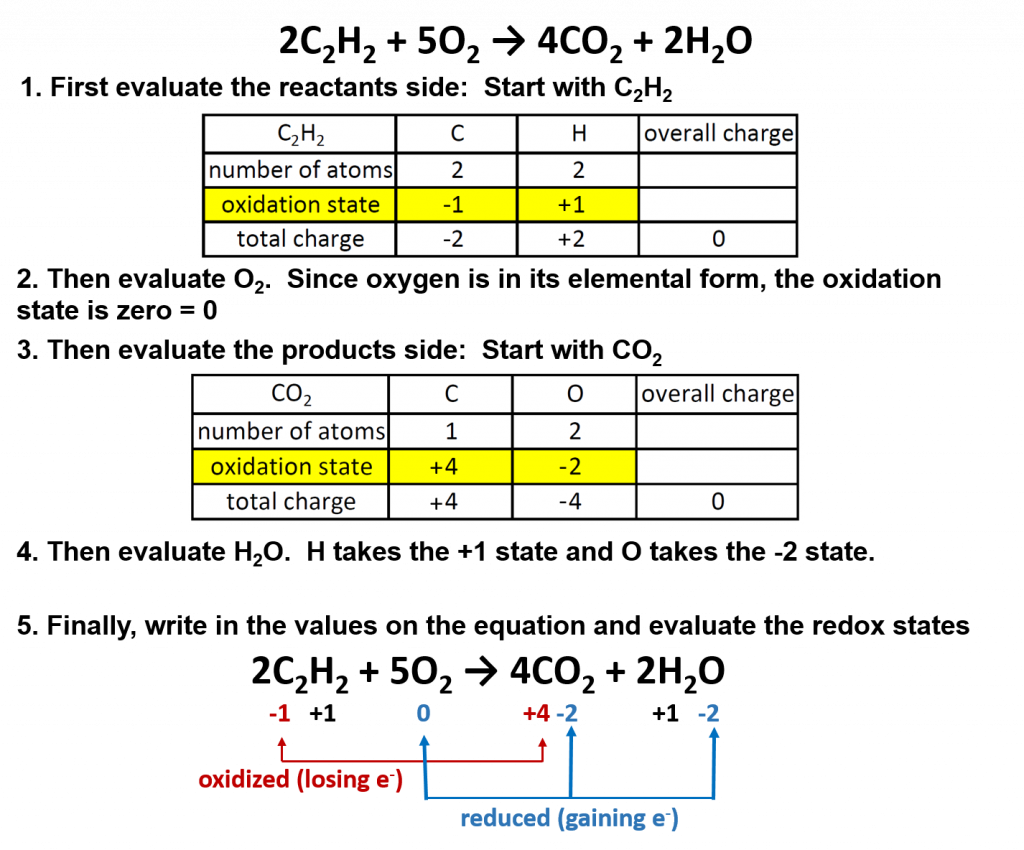
One example is the burning of acetylene (C2H2) in torches:
2C2H2 + 5O2 → 4CO2 + 2H2O
Oxygen (in its elemental form) is a crucial reactant in combustion reactions, and it is also present in the products. Combustion reactions can be evaluated for their redox potential, as we did for the thermite reaction above.
Overall in combustion reactions, the hydocarbon (in this case, acetylene) is oxidized by molecular oxygen to produce carbon dioxide and water. In the process, oxygen is reduced.
In respiration, the biochemical process by which the oxygen we inhale in air oxidizes foodstuffs to carbon dioxide and water, redox reactions provide energy to living cells. A typical respiratory reaction is the oxidation of glucose (C6H12O6), a simple sugar.
C6H12O6 + 6O2 → 6CO2 + 6H2O
A Closer Look at the Importance of Redox Reactions
Organic chemists use a variety of redox reactions. For example, potassium dichromate (K2Cr2O7) is a common oxidizing agent that can be used to oxidize alcohols (symbolized by the general formula R-OH, where R can be any group, and the OH represents the alcohol functional group). The product of the reaction depends on the location of the OH functional group in the alcohol molecule, the relative proportions of alcohol and the dichromate ion, and reaction conditions such as temperature. If the OH group is attached to a terminal carbon atom and the product is distilled off as it forms, the product is an aldehyde, which has a terminal carbonyl group (C=O). One example is the reaction used by the Breathalyzer to detect ethyl alcohol (C2H5OH) in a person’s breath:
3C2H5OH + Cr2O72− + 8H+ → 3CH3CHO + 2Cr3+ + 7H2O
Figure 5.3 Drinkers Learn Their Limit Using a Standard Breathalyzer Test
Picture from: KOMUnews
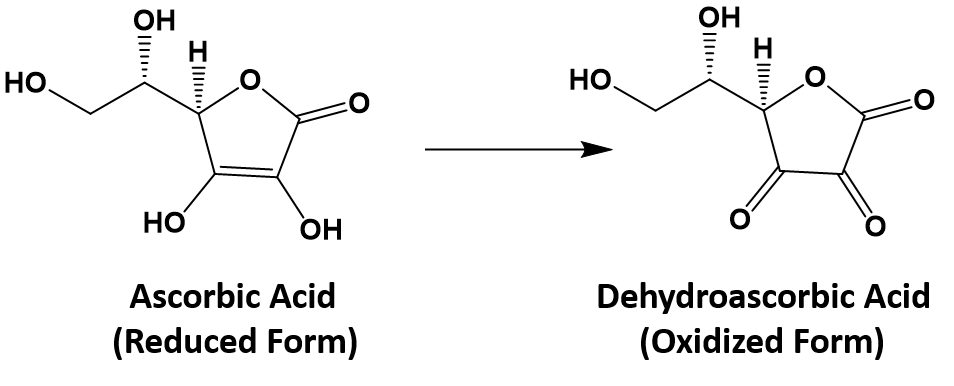
In food chemistry, the substances known as antioxidants are reducing agents. Ascorbic acid (also known as vitamin C; C6H8O6) is thought to prevent potentially damaging oxidation of living cells. In the process, it is oxidized to dehydroascorbic acid (C6H6O6).
In the stomach, ascorbic acid reduces the nitrite ion (NO2−) to nitric oxide (NO):
C6H8O6 + 2H+ + 2NO2− → C6H6O6 + 2H2O + 2NO
If this reaction did not occur, nitrite ions from foods would oxidize the iron in hemoglobin, destroying its ability to carry oxygen.
Tocopherol (vitamin E) is also an antioxidant. In the body, vitamin E is thought to act by scavenging harmful by-products of metabolism, such as the highly reactive molecular fragments called free radicals. In foods, vitamin E acts to prevent fats from being oxidized and thus becoming rancid.
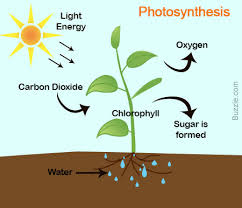
Finally, and of greatest importance, green plants carry out the redox reaction that makes possible almost all life on Earth. They do this through a process called photosynthesis, in which carbon dioxide and water are converted to glucose (C6H12O6). The synthesis of glucose requires a variety of proteins called enzymes and a green pigment called chlorophyll that converts sunlight into chemical energy (Figure 5.4). In this reaction, carbon dioxide is reduced to form glucose (C6H12O6), and water is oxidized to oxygen gas. Other reactions convert the glucose (C6H12O6) to more complex carbohydrates, plant proteins, and oils. The chemical reaction that occurs is as follows:
6CO2 + 6H2O → C6H12O6 + 6O2
Figure 5.4 Diagram of Photosynthesis. In photosynthesis, light energy from the sun is harvested by the chlorophyll pigments primarily in the plant leaves where it is used to convert carbon dioxide and water into sugar (glucose).
Image provided from: Masroor.nida.ns
5.5 Chapter Summary
To ensure that you understand the material in this chapter, you should review the meanings of the following bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
Scientific laws are general statements that apply to a wide variety of circumstances. One important law in chemistry is the law of conservation of matter, which states that in any closed system, the amount of matter stays constant.
Chemical equations are used to represent chemical reactions. Reactants change chemically into products. The law of conservation of matter requires that a proper chemical equation be balanced. Coefficients are used to show the relative numbers of reactant and product molecules.
In stoichiometry, quantities of reactants and/or products can be related to each other using the balanced chemical equation. The coefficients in a balanced chemical reaction are used to devise the proper ratios that relate the number of molecules of one substance to the number of molecules of another substance.
Chemical reactions can be classified by type. Combination reactions (also called synthesis reactions) build or make a substance from other substances, whereas, decomposition reactions break one substance down into multiple substances.
Single replacement (or displacement) reactions occur when an element (most frequently a metal) replaces another element from a compound. They can occur in two forms: cationic exchange or anionic exchange. In cationic exchanges, the positive ions (cations) of the equation change place. In anionic exchanges, the negative ions (anions) change place. Note that single replacement reactions are often also redox reactions.
Double replacement (or displacement) reactions occur when two chemical groups substitute for each other (change partners) in their respective compounds. Double replacement reactions can be of two major types: (1) precipitation reactions wherein, at least one of the products forms a solid precipitate during the reaction, and (2) acid-base neutralization reactions, wherein an acid is neutralized by a base to form a salt and water. The solubility rules are used to determine if products in a double displacement reaction will be insoluble and precipitate from the reaction mixture. Both precipitation and acid-base neutralization reactions can be written as net ionic equations.
Oxidation reactions are reactions in which an atom loses an electron. Reduction reactions are reactions in which an atom gains an electron. These two processes always occur together, so they are collectively referred to as oxidation-reduction (or redox) reactions. The species being oxidized it called the reducing agent, while the species being reduced is the oxidizing agent. Combustion reactions are a type of redox reaction that combine molecular oxygen with the atoms of another reactant.
Oxidation-reduction reactions are common in organic and biological chemistry. Respiration, the process by which we inhale and metabolize oxygen, is a series of redox reactions. In the absence of oxygen, redox reactions still occur in a process called anaerobic metabolism. Antioxidants such as ascorbic acid also play a part in the human diet, acting as reducing agents in various biochemical reactions. Photosynthesis, the process by which plants convert water and carbon dioxide to glucose, is also based on redox reactions.
5.6 References
- UC Davis, Libretexts. (2016) Chemistry. https://chem.libretexts.org/Core/Inorganic_Chemistry/Descriptive_Chemistry/Main_Group_Reactions/Reactions_in_Aqueous_Solutions/Precipitation_Reactions
- Anonymous. (2012) Introduction to Chemistry: General, Organic, and Biological (V1.0). Published under Creative Commons by-nc-sa 3.0. Available at: http://2012books.lardbucket.org/books/introduction-to-chemistry-general-organic-and-biological/index.html