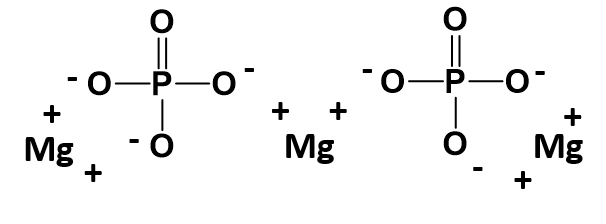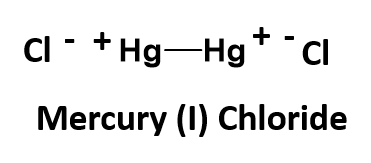Home » Student Resources » Online Chemistry Textbooks » CH150: Preparatory Chemistry » CH150: Chapter 3 – Ions and Ionic Compounds
MenuCH150: Preparatory Chemistry
CH150: Chapter 3 – Ions and Ionic Compounds
This content can also be downloaded as an printable PDF, adobe reader is required for full functionality.
This text is published under creative commons licensing, for referencing and adaptation, please click here.
3.1 Introduction to the Octet Rule
3.2 Ions and the Periodic Table
Common Cations
Common Anions
Ions of Transition Metals
3.3 Ionic Bonding
3.4 Practice Writing Correct Ionic Formulas
3.5 Naming Ions and Ionic Compounds
3.6 Polyatomic Ions
3.7 Naming Polyatomic Ions
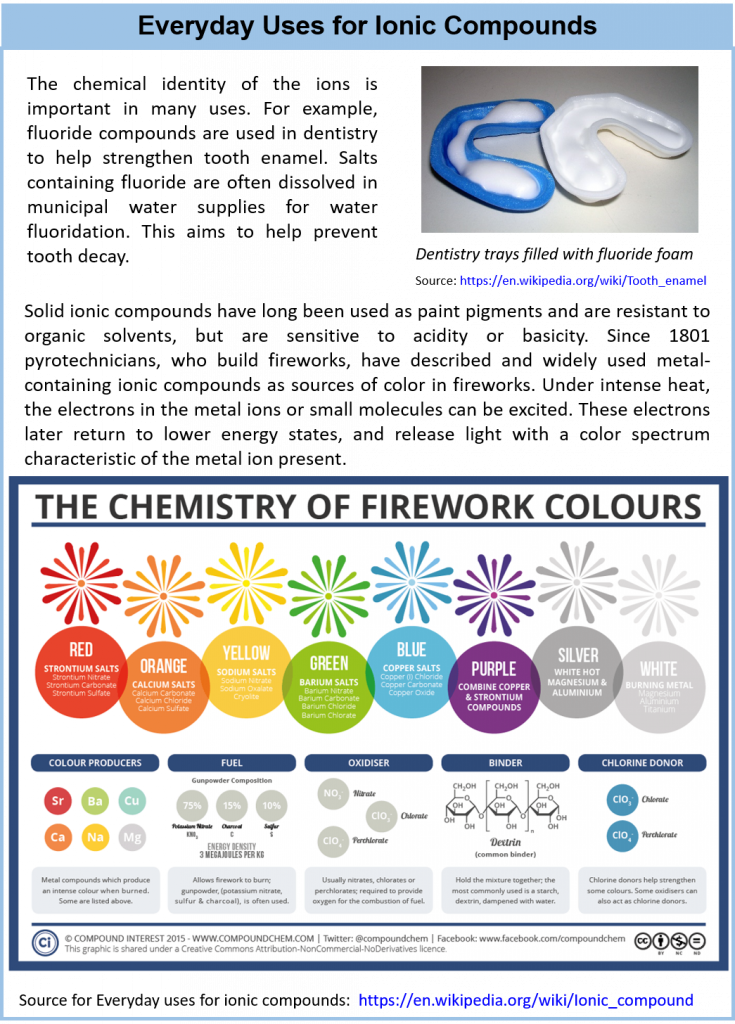
3.8 Properties and Types of Ionic Compounds
3.9 Arrhenius Acids and Bases
3.10 Chapter Summary
3.11 References
3.1 Introduction to the Octet Rule
Up until now we have been discussing only the elemental forms of atoms which are neutrally charged. This is because the number of electrons (negative in charge) is equal to the number of protons (positive in charge). The overall charge on the atom is zero, because the magnitude of the negative charge is the same as the magnitude of the positive charge. This one-to-one ratio of charges is not, however, the most common state for many elements. Deviations from this ratio result in charged particles called ions.
Throughout nature, things that are high in energy tend to move toward lower energy states. Lower energy configurations are more stable, so things are naturally drawn toward them. For atoms, these lower energy states are represented by the noble gas elements. These elements have electron configurations characterized by full s and p subshells. This makes them stable and unreactive. They are already at a low energy state, so they tend to stay as they are.
The elements in the other groups have subshells that are not full, so they are unstable when compared to the noble gases. This instability drives them toward the lower energy states represented by the noble gases that are nearby in the periodic table. In these lower energy states, the outermost energy level has eight electrons (an “octet”). The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the “Octet Rule.”
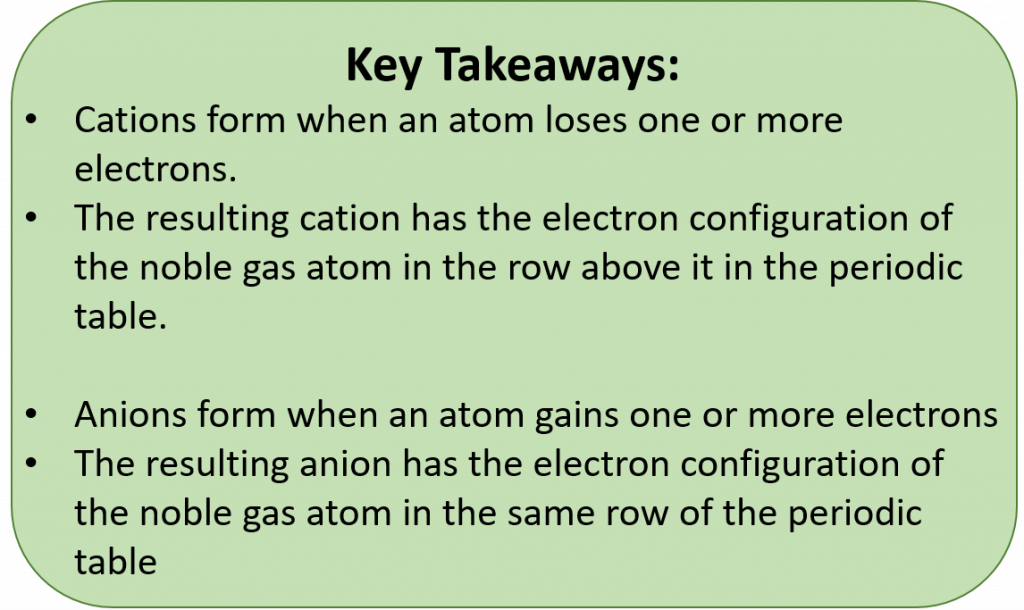
There are two ways for an atom that does not have an octet of valence electrons to obtain an octet in its outer shell. One way is the transfer of electrons between two atoms until both atoms have octets. Because some atoms will lose electrons and some atoms will gain electrons, there is no overall change in the number of electrons, but with the transfer of electrons the individual atoms acquire a nonzero electric charge. Those that lose electrons become positively charged, and those that gain electrons become negatively charged. Recall that atoms carrying positive or negative charges are called ions. If an atom has gained one or more electrons, it is negatively charged and is called an anion. If an atom has lost one or more electrons, it is positively charged and is called a cation. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds.
The second way for an atom to obtain an octet of electrons is by sharing electrons with another atom. These shared electrons simultaneously occupy the outermost shell of both atoms. The bond made by electron sharing is called a covalent bond. Covalent bonding and covalent compounds will be discussed in Chapter 4 “Covalent Bonding and Simple Molecular Compounds”.
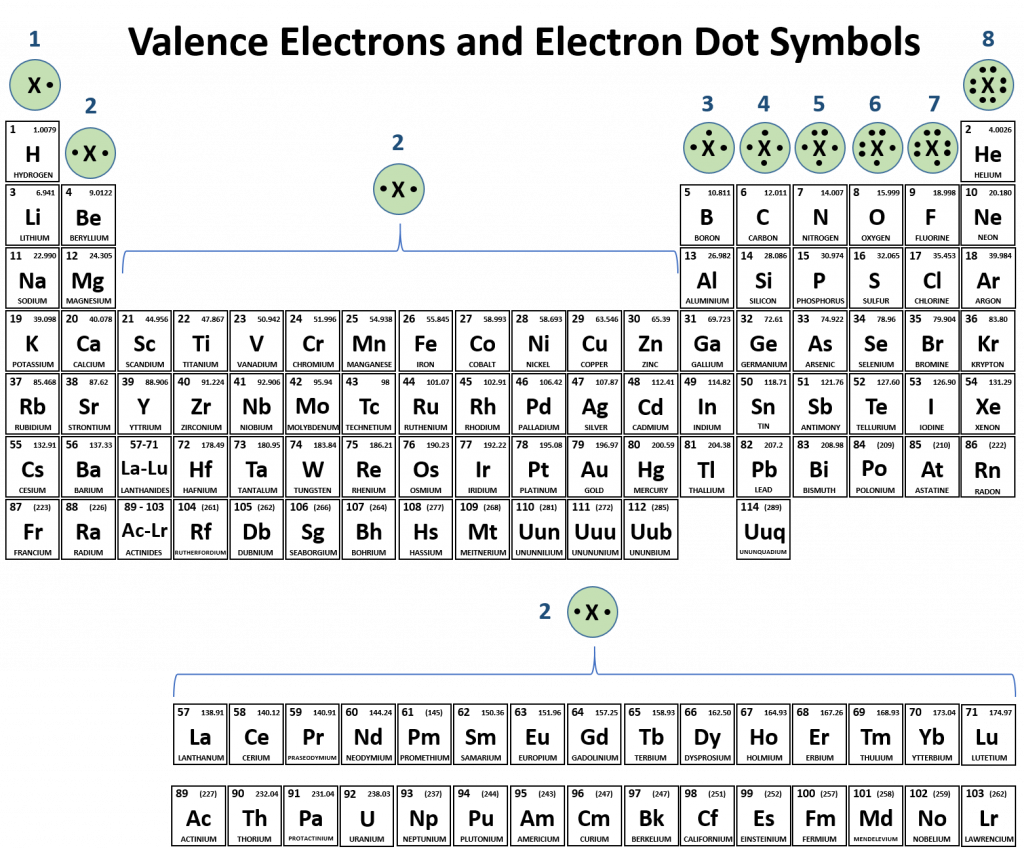
At the end of chapter 2, we learned how to draw the electron dot symbols to represent the valence electrons for each of the elemental families. This skill will be instrumental in learning about ions and ionic bonding. Looking at Figure 3.1, observe the Noble Gas family of elements. The electron dot symbol for the Nobel Gas family clearly indicates that the valence electron shell is completely full with an octet of electrons. If you look at the other families, you can see how many electrons they will need to gain or lose to reach the octet state. Above, we noted that elements are the most stable when they can reach the octet state. However, it should also be noted that housing excessively high negative or positive charge is unfavorable. Thus, elements will reach the octet state and also maintain the lowest charge possible. You will note that for the IA, IIA, IIIA and transition metals groups, it is more economical to lose electrons (1-3 electrons) from their valence shells to reach the octet state, rather than to gain 5-7 electrons. Similarly main group columns VA, VIA, and VIIA tend to gain electrons (1-3) to complete their octet, rather than losing 5-7 electrons. Some atoms, like carbon, are directly in the middle. These atoms don’t like to gain or lose electrons, but tend to favor the sharing model of chemical bonding. The remaining sections of this chapter will focus on the formation of ions and the resulting ionic compounds.
Figure 3.1 Periodic Table with Electron Dot Symbols.
Figure 3.2 Ionization Within and Electric Field. (A) Depiction of St. Elmo’s Fire at the tips of a ship’s masts. (B) In many high voltage applications plasma ionization is an unwanted side effect. Shown is a long exposure photograph of corona discharge on an insulator string of a 500 kV overhead power line. This type of plasma discharge represent a significant power loss for electric utilities.
Photograph depicted in a (A) by: Unknown Author
Photograph depicted in a (B) by: Nitromethane
3.2 Ions and the Periodic Table
The elements on the right side of the periodic table, nonmetals, gain the electrons necessary to reach the stable electron configuration of the nearest noble gas. Elements on the left side of the periodic table, metals, lose the electrons necessary to reach the electron configuration of the nearest noble gas. Transition elements can vary in how they move toward lower energy configurations.
Common Cations
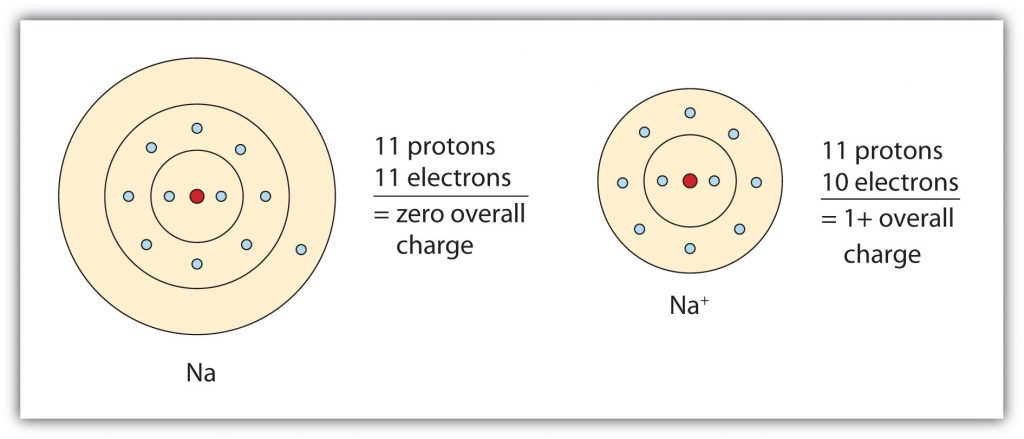
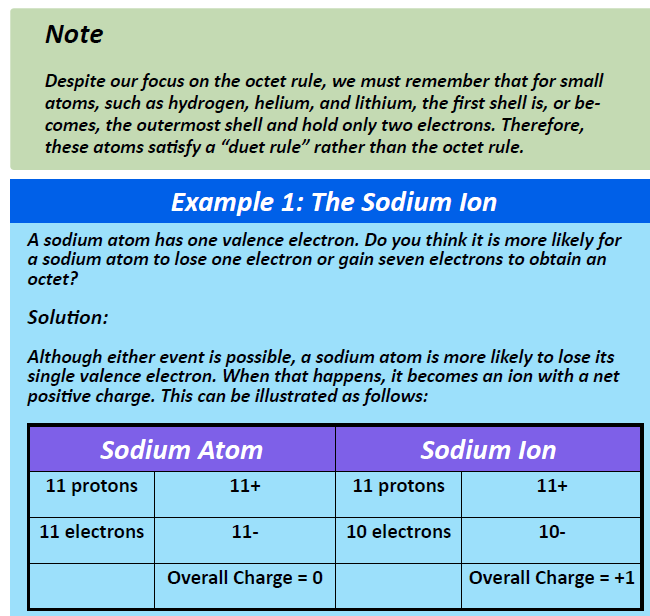
Group IA elements form ions with a +1 charge. They lose one electron upon ionization, moving into the electron configuration of the previous noble gas. For example as shown in Figure 3.3, when a sodium (Na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (Na+) with the electron configuration that looks like the previous noble gas, neon. The sodium ion has one fewer electron than it has protons, so it has a single positive charge and is called a cation.
Figure 3.3 The Formation of a Sodium Ion. Sodium tends to lose it’s valence shell electron in the third shell during ionic bond formation. It is left with a full octet in the second shell and now has the electron configuration of neon. Note that it still has the same number of protons (11) as the original sodium atom and retains the identity of sodium. However, there are now only 10 electrons within the electron cloud, resulting in a net positive (+1) charge.
Upon losing that electron, the sodium ion now has an octet of electrons from the second principal energy level. The equation below illustrates this process.
Na → Na+ + e−
1s22s22p63s1 1s22s22p6(octet)
The electron configuration of the sodium ion is now the same as that of the noble gas neon. The term isoelectronic refers to an atom and an ion of a different atom (or two different ions) that have the same electron configuration. The sodium ion is isoelectronic with the neon atom. Consider a similar process with magnesium and with aluminum:
Mg → Mg2+ + 2e−
1s22s22p63s2 1s22s22p6(octet)
Al → Al3+ + 3e−
1s22s22p63s23p1 1s22s22p6(octet)
In this case, the magnesium atom loses its two valence electrons in order to achieve the same noble-gas configuration. The aluminum atom loses its three valence electrons. The Mg2+ ion, the Al3+ ion, the Na+ ion, and the elemental Ne atom are all isoelectronic. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Only larger atoms, such as lead and uranium, can typically carry larger charge states.
Overall, Group IIA elements lose two valence electrons to reach the electron configuration of the noble gas preceding them in the periodic table and Group IIIA elements lose three electrons to form ions with a +3 charge. This gives them the electron configuration of the noble gas that comes before them in the periodic table.
While hydrogen is in the first column, it is not considered to be an alkali metal, and so it does not fall under the same classification as the elements below it in the periodic table. This is because hydrogen only has an s-subshell and can only house a total of 2 electrons to become filled and obtain the electron configuration of helium. Thus, instead of following the octet rule, it reaches greater stability by gaining a “duet” of electrons through bonding with other atoms. Thus, hydrogen can form both covalent bonds and ionic bonds, depending on the element that it is interacting with. When it participates in ionic bonds, it most often will lose its electron forming a +1 cation. Note, that hydrogen only has one electron to begin with, so when it loses an electron in the ionized state, there is only a single proton left in the nucleus of the atom. Thus, when hydrogen is ionized to H+ it is often referred to as a proton. It can also be ionized, forming a -1 anion. In this case, the H– anion is named using standard convention forming the hydride ion. During the ionization of hydrogen, the H+ state is more common than the H– state. In addition, the H+ ion is very important in the chemistry of acids. Acids are defined as compounds that donate H+ ions in aqueous solutions, and will be discussed in more detail in Chapter 9.
Common Anions
Elements on the other side of the periodic table, the nonmetals, tend to gain electrons in order to reach the stable electron configurations of the noble gases that come after them in the periodic table.
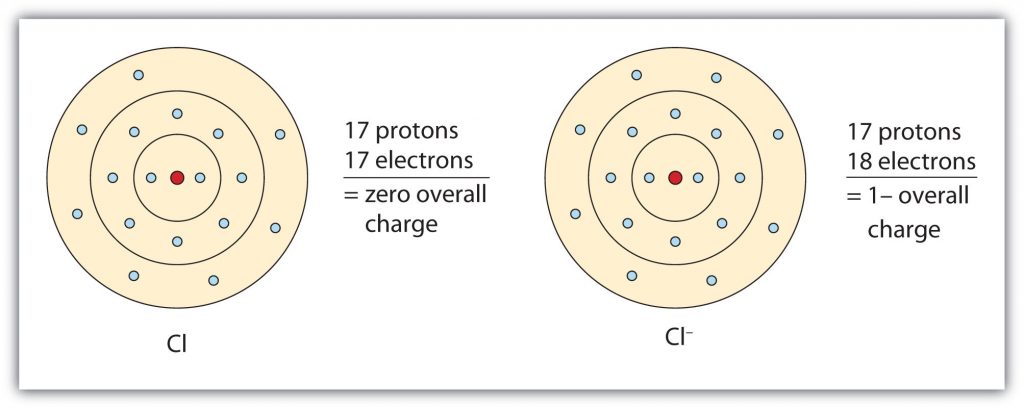
Group VIIA elements gain one electron when ionized, obtaining a -1 charge. For example as shown in Figure 3.4, chlorine (Cl), when ionized, gains an electron to reach the electron configuration of the noble gas that follows it in the periodic table, argon. This gives it a single negative charge, and it is now a chloride ion (Cl–); note the slight change in the suffix (-ide instead of -ine) to create the name of this anion.
Fig 3.4 The Formation of a Chloride Ion. On the left, a chlorine atom has 17 electrons. On the right, the chloride ion has gained an extra electron for a total of 18 electrons and a 1– charge. Note that the chloride ion has now filled its outer shell and contains eight electrons, satisfying the octet rule.
Group VIA elements gain two electrons upon ionization, obtaining -2 charges and reaching the electron configurations of the noble gases that follow them in the periodic table. Whereas, Group VA elements gain three electrons, obtaining -3 charges and also reaching the electron configurations of the noble gases that follow in the periodic table.
When nonmetal atoms gain electrons, they often do so until their outermost principal energy level achieves an octet. This process is illustrated below for the elements fluorine, oxygen, and nitrogen.
F + e− → F−
1s22s22p5 1s22s22p6(octet)
O + 2e− → O2−
1s22s22p4 1s22s22p6(octet)
N + 3e− → N3−
1s22s22p3 1s22s22p6(octet)
All of these anions are isoelectronic with each other and with neon. They are also isoelectronic with the three cations from the previous section. Under typical conditions, three electrons is the maximum that will be gained in the formation of anions.
It is important not to misinterpret the concept of being isoelectronic. A sodium ion is very different from a neon atom because the nuclei of the two contain different numbers of protons. One is an essential ion that is a part of table salt, while the other is an unreactive gas that is a very small part of the atmosphere. Likewise, sodium ions are very different than magnesium ions, fluoride ions, and all the other members of this isoelectronic series (N3−,O2−,F−,Ne,Na+,Mg2+,Al3+)
.
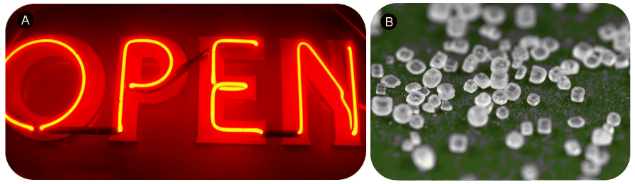
Figure 3.5: Neon gas (A) and sodium chloride crystals (B). Neon atoms and sodium ions are isoelectronic. Neon is a colorless and unreactive gas that glows a distinctive red-orange color in a gas discharge tube. Sodium ions are most commonly found in crystals of sodium chloride, ordinary table salt.
Ions of Transition Metals
The transition metals are an interesting and challenging group of elements. They have perplexing patterns of electron distribution that don’t always follow the electron filling rules. Predicting how they will form ions is also not always obvious.
Transition metals belong to the d-block, meaning that the d-subshell of electrons is in the process of being filled with up to ten electrons. Many transition metals cannot lose enough electrons to attain a noble-gas electron configuration. In addition, the majority of transition metals are capable of adopting ions with different charges. Iron, which forms either the Fe2+ or Fe3+ ions, loses electrons as shown below.
Fe → Fe2+ + 2e−
[Ar]3d64s2 [Ar]3d6
Fe → Fe3+ + 3e−
[Ar]3d64s2 [Ar]3d5
According to the Aufbau process, the electrons fill the 4s sublevel before beginning to fill the 3d sublevel. However, the outermost s electrons are always the first to be removed in the process of forming transition metal cations. Because transition metals have two valence electrons, the charge of 2+ is a very common one for their ions. This is the case for iron above. In addition to the 2+ state, iron can also form a 3+ cation. This is because a half-filled d subshell (d5) is particularly stable, which is the result of an iron atom losing a third electron.
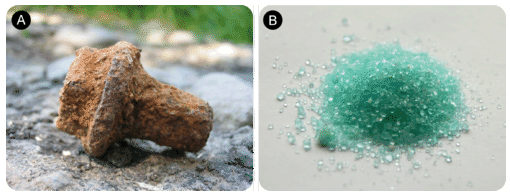
Figure 3.6 (A). Rust is a complex combination of oxides of iron, among them iron (III) oxide, Fe2O3. (B) Iron (II) sulfate, FeSO4 is an example of a compound that contains iron in the 2+ cationic state. It has been known since ancient times as green vitriol and was used for centuries in the manufacture of inks.
Some transition metals that have relatively few d electrons may attain a noble gas electron configuration. Scandium is an example.
Others may attain stable configurations with a full d-subshell, such as zinc and copper.
The resulting configuration above, with 18 electrons in the outermost principal energy level, is referred to as a pseudo noble-gas electron configuration. It gives particular stability to the Zn2+ and Cu+ ions.
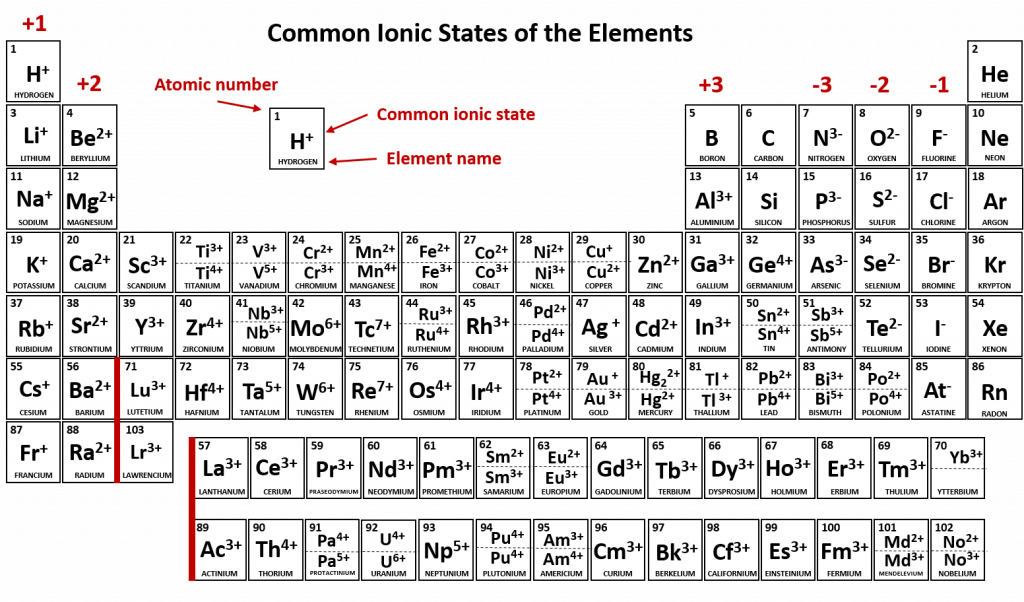
Figure 3.7 depicts the most common ionic states of the elements and shows the two most common ionic states for elements that can form more than one ion.
Figure 3.7 Common Ionic States of the Elements. For elements that have more than one common ionic state, both states are listed. Note that when mercury carries a +1 charge, it forms an uncommon polyatomic ionic state, Hg22+ where two Hg atoms share electrons and then each also have a +1 charge state (see section XX for more details about polyatomic ions and Hg22+). For the printable PDF version of this table (with the common polyatomic ions), click the link below:
3.3 Ionic Bonding
Most of the rocks and minerals that make up the Earth’s crust are composed of positive and negative ions held together by ionic bonding. An ionic compound is an electrically neutral compound consisting of positive and negative ions. You are very familiar with some ionic compounds such as sodium chloride (NaCl). A sodium chloride crystal consists of equal numbers of positive sodium ions (Na+) and negative chloride ions (Cl−).
Anions and cations have opposing charges. Because of this, they are attracted to one another. When an anion and a cation are drawn together due to this electrostatic attraction, they can form an ionic bond. This kind of bond is the result of opposing charges attracting one another, and is distinct from other types of bonding. Two or more ions bound by electrostatic attraction make an ionic compound. The simplest ionic compounds are binary ionic compounds or those that only contain two atoms, one acting as the cation, and one acting as the anion. Thus, we will focus on the formation of binary ionic compounds first.
Sodium chloride, or table salt, is an ionic compound. Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. The chloride ion has one excess electron, giving it a -1 charge. The result of this electron transfer is that the sodium cation and chloride anion become bound through electrostatic attraction, forming sodium chloride, an ionic compound. Note that, electrons cannot be simply “lost” to nowhere in particular, they always end up going to another atom or molecule. Ionic reactions can be represented by electron dot diagrams, as shown below for sodium chloride.
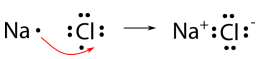
The ionic bond is the attraction of the Na+ ion for the Cl− ion. It is conventional to show the cation without dots around the symbol to emphasize that the original energy level that contained the valence electron is now empty. The anion is now shown with a complete octet of electrons. The final formula for sodium chloride is NaCl. Notice that both ions are represented but their charges are not shown. This is because within ionic compounds the overall charge on the compound is zero, i.e. the charge states of the cation(s) and the anion(s) involved in the bond need to be paired in such a way that the number of positive charges equals the number of negative charges. For sodium chloride this is an easy task as one chloride ion has a -1 charge and one sodium ion has a positive charge +1, cancelling each other to zero. Also note that in chemical formulas that the cation always comes first and the anion is always placed second in the formula.
For a compound such as magnesium chloride, it is not quite as simple. Because magnesium has two valence electrons, it needs to lose both to achieve the noble-gas configuration. Therefore, two chlorine atoms will be needed.
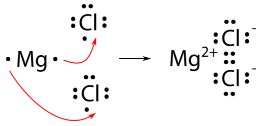
The final formula for magnesium chloride is MgCl2. Note that the subscript (2) next to the chloride ion, indicates that there are two chloride ions paired with each magnesium cation. When there is only one ion present in a formula, (i.e the magnesium ion in this case), the subscript of one is implied instead of shown in the formula. As in the case of NaCl, there are no charges shown in the final formula of MgCl2. This is because the positive charge of the magnesium ion (+2) is balanced by the negative charge of the two chloride ions [2 X (-1) = -2] giving the overall molecule a net charge of zero.
3.4 Practice Writing Correct Ionic Formulas
To predict and write correct chemical formulas, the key fundamental steps that are required, are (1) knowing the charge states of the ions and (2) using basic math to help you determine how many cations and anions are needed to reach a zero charge state, (3) writing the chemical forumulas with the cation first followed by the anion, and (4) writing the formula with the lowest ratio of cations and anions to create a net neutral compound.
Overall, ionic bonding occurs between a cation (electron donor) and an anion (electron acceptor) to form a compound that has an overall neutral net charge. Of note, ionic bonds usually occur between a metal and a nonmetal. This will help you recognize ionic compounds more easily, once we learn about covalent bonding (which occurs most commonly between two nonmetals, or between a nonmetal and a semimetal (metalloid).
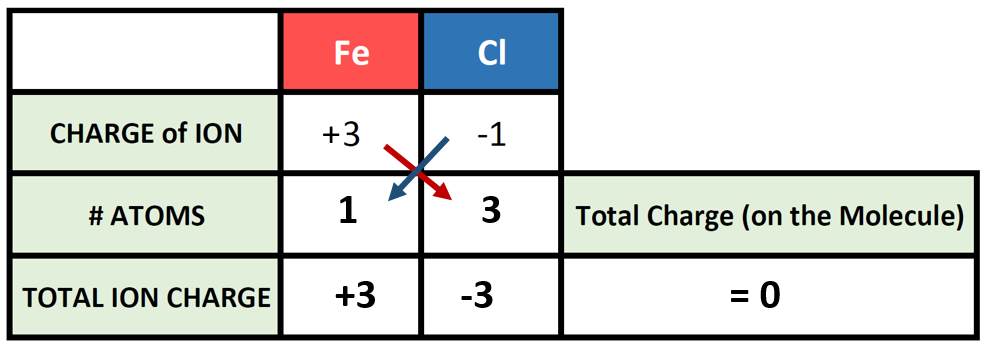
So, say we want to write the correct chemical formula for a molecule that contains Fe3+ as the cation, and Cl– as the anion. What is the correct ionic formula?
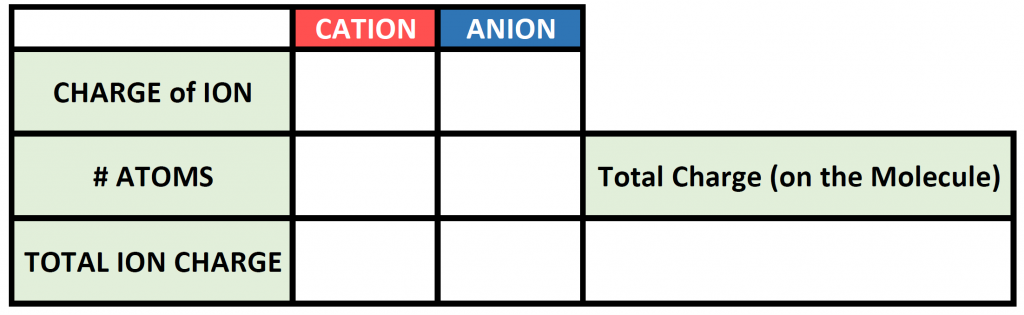
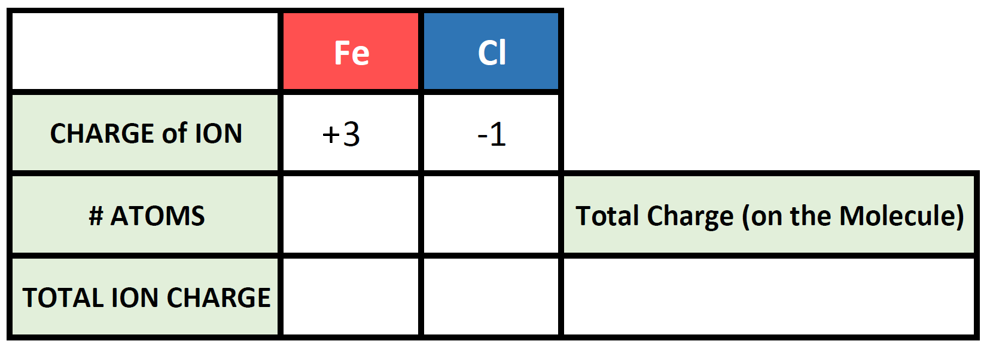
To begin this type of problem, I recommend drawing out a charge box or a charge table to help you keep track of the number of ions used, the charges of those ions, and the overall positive and negative charges on the molecule. Drawing out the electron dot symbols can also be helpful. Here is an example of a generic charge box
Let’s try it out for our example of Fe3+ and Cl–. First, let’s fill in what we know about each element and it’s ionic state:
So now we have our charge box set up with our known information. Now we need to figure out how many atoms of the cation and the anion are required to cancel out the overall positive and negative charge on the resulting molecule. To do this, it is often useful to use the cross-multiplication strategy, where you try using the charge number for the cations, as the number of atoms of anion required, and the charge number for the anion as the number of atoms of the cation required. Multiply each of the ion charges by the number of atoms to calculate the total ion charges of the cation(s) and anion(s) present and then add these numbers together to find the total charge on the compound. This will usually get you to the stable ionic formula that has a net neutral charge of zero.
The # of atoms column then becomes the subscripts that you need to use to construct the correct ionic formula. In this case 1 atom of iron (Fe) with 3 atoms of chlorine (Cl) for a formula of FeCl3.
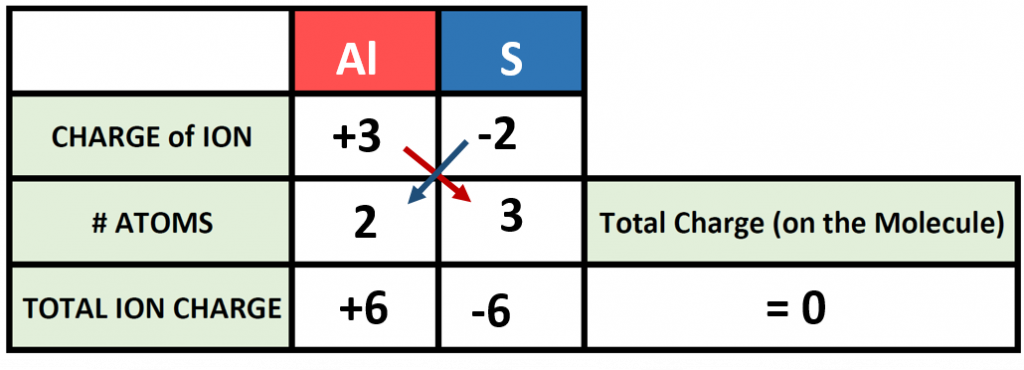
The previous example is pretty straight forward, and you may have been able to construct the formula in you head. However, as the complexity of formula making increases, it is good to be able to use the charge box method to double check your work. For example, what would the correct ionic formula be for aluminum sulfide? First, identify the two atoms involved (Aluminum and Sulfur) and start building your charge box with what you know from the periodic table. From the periodic table in Figure 3.7, you can see that aluminum forms a cation with a +3 charge whereas sulfur forms an anion with a -2 charge state.
For step 1: Add in the correct charge for the cation and anion in question, in this case +3 for Al and -2 for S. For Step 2: Use the cross multiply rule to predict how many atoms will be needed from each type and multiply through the total ion charge for both the cation and anion. For Step 3: Add the products together to be sure that your compound is stable and the net charge on the formula is zero. Step 4: Use the # Atoms value to create the subscripts for your chemical formula. In our example, we require 2 atoms of Al and 3 atoms of S. This would be written as Al2S3 as the final product.
3.5 Naming Ions and Ionic Compounds
Some compounds have common names, like water for H2O. However, there are thousands of other compounds that are uncommon or have multiple names. Also, the common name is usually not recognized internationally. What looks like water to you might look like agua or vatten to someone else. To allow chemists to communicate without confusion, there are naming conventions to determine the systematic name of a chemical. For the chemistry naming system in this text, we will primarily be using the International Union of Pure and Applied Chemistry (IUPAC) naming system. Note that there is also an older and more archaic (-ous and -ic) naming system, in addition to the IUPAC system. In some instances the older naming system is still in high use. These deviations from the IUPAC system will be noted throughout the text, as you will likely still see this older nomenclature still in use within chemical laboratories and the health sciences field.
The convention for naming cations is very easy. It is simply to take the element name and add the term ‘ion’ to the end of it. So if we are referring to a sodium atom that has lost one electron (Na+), we would use the term sodium ion. This indicates that sodium is in the +1 charge state, rather than the elemental form of sodium (which has an equal number of protons and electrons and is neutral in charge). Using the ion naming system when referring to ions, rather than the elemental names of atoms is important, as the reactivity of the ion vs the elemental form of a substance can be quite different. For example, if you add the sodium ion to your glass of drinking water in the form of NaCl (or table salt), you will have a nice salty drink on your hands. On the other hand, if you add the elemental form of sodium to your glass of drinking water, it will explode in your face, as the elemental form of sodium is very reactive with water!
For cations that have more than one charge state the name of the atom is followed by a roman numeral and then the term ion, to distinguish the different ionic states. For example, iron has two predominant ionic forms, Fe2+ and Fe3+. Thus, in naming these two ions, we would refer to the first one as the iron (II) ion, and the second as the iron (III) ion. This way, there is no confusion about which ion is being referred to when discussing a compound.
Naming anions is a little more complicated. The ending of the element is typically dropped and replaced with the ‘ide’ ending followed by the term ion. For example, Cl– is referred to as the chloride ion, rather than the chlorine ion. In this case, the ‘-ine’ ending of chlorine is dropped and replaced with the ‘ide’ ending. For sufur, the ‘-ur’ ending is dropped and replaced with ‘ide’ to form the sulfide ion. Similarly phosphorus is converted to the phosphide ion, nitrogen to the nitride ion, and oxygen to the oxide ion. The ‘-ide’ ending is useful because it helps the listener distinguish very quickly between the different types of ions being discussed (the cation which retains the element name vs. the anion which changes the elemental name to the ‘-ide’ ending).
When naming ionic compounds the term ion is dropped and the cation and anion names are placed together, with the cation always listed first and the anion listed last. If the elements involved in the ionic bond only have one possible ionic state, no roman numerals are needed in the name. For example, when the Na+ and the Cl– come together to make NaCl, the resulting compound is called sodium chloride. Similary, if Mg2+ and Cl– come together to make MgCl2, the resulting compound is called magnesium chloride. However, if the elements involved in the ionic bond have more than one possible ionic state, the roman numeral system is used to clarify which ion is participating in the bond. For example, if Fe3+ and Cl– come together to form FeCl3, we will need to distinguish it from Fe2+ coming together with Cl– to form FeCl2 in the name so that everyone will understand which ion of iron is being referred to in the reaction. In this case, the first compound will be called iron (III) chloride, and the second compound is iron (II) chloride.
The key feature about naming ionic compounds is that you should be able to draw the structure from the name, and that you should be able to create the name from the structure. Let’s do some practice!
3.6 Polyatomic Ions
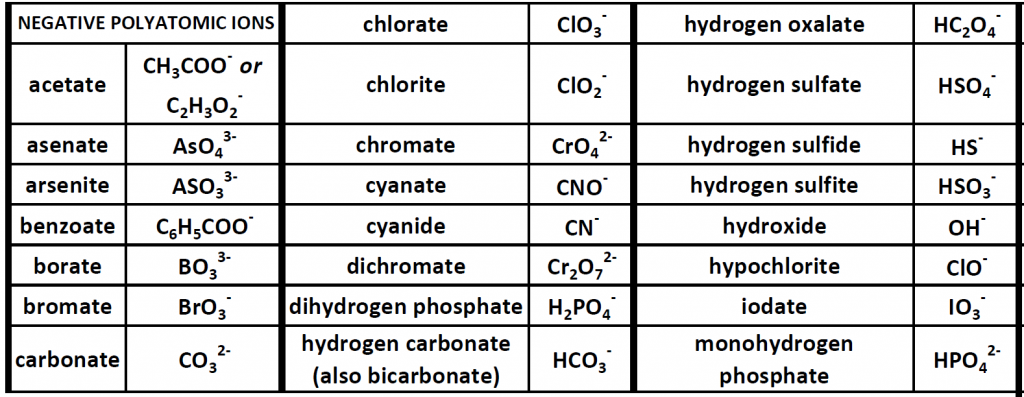
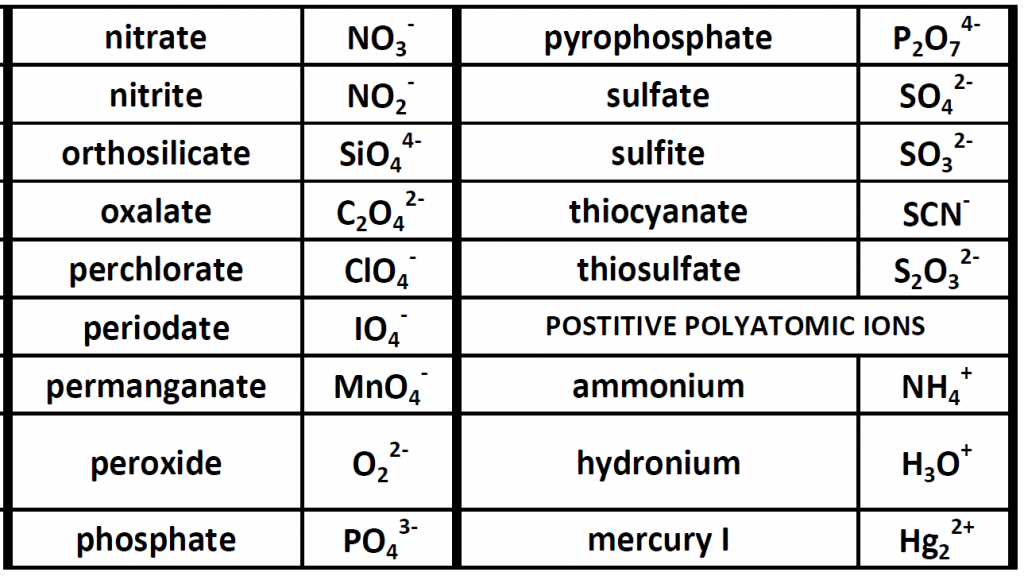
Up until now, we have been looking at compounds involving monoatomic ions, or ions that occur with a single atom. However, many commonly found ions are composed of multiple atoms that are bound to one another through the sharing of electrons, or covalently. These ions behave as a single unit, bearing a charge and interacting with other ions and compounds just like the monatomic ions discussed above. Because these ions are made of multiple atoms, they are called polyatomic ions. It is more common for polyatomic ions to be negatively charged than to be positively charged. Below is a chart showing some commonly encountered polyatomic ions.
Table 3.1 Common Polyatomic Ions
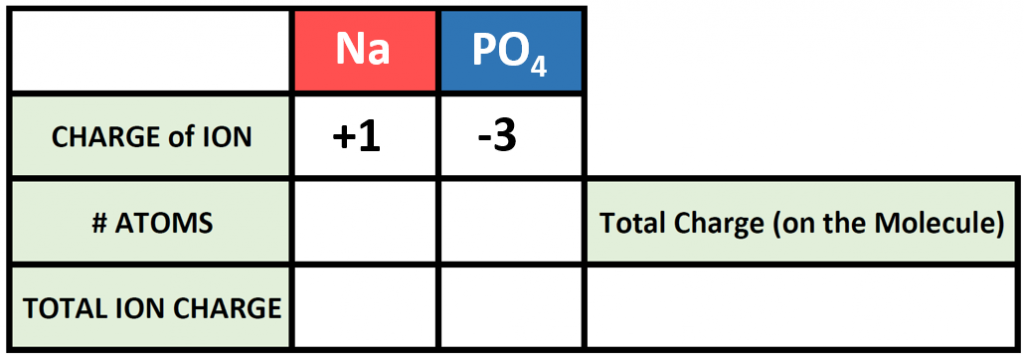
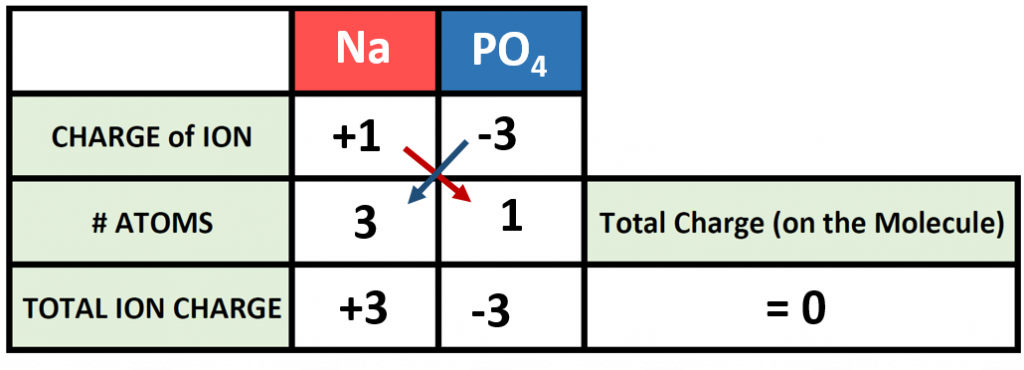
Polyatomic ions can be thought of in a very similar way to monoatomic ions, in that they are ionized by either gaining or losing electrons so that they carry a charge. If they gain electrons, they will become an anion and carry a negative charge, and if they lose electrons, they will become a cation and carry a positive charge. The charge of a polyatomic ion is represented as a supercript that is placed at the upper righthand edge of the ion. For example, for the phosphate ion, the chemical formula is PO43-. This indicates that the overall -3 charge is distributed to the entire PO4 molecule, and that when it is involved in forming an ionic compound, the entire PO43- ion moves as and is treated as a single unit. Let’s try making a few compounds using phosphate as an example. First let’s build a molecule of sodium phosphate. Note that when you are asked to build molecules from their name, you can often recognize when you have a polyatomic ion due to the name. Recall that monoatomic anions end in the suffix ‘-ide’. Thus, when you see a different suffix ending, such as ‘-ate’ or ‘-ite’, this should indicate that you are dealing with a polyatomic ion and you should refer to the table above to help you discern the correct ion formula to use. For the sodium phosphate example, we can build this molecule using the same charge box diagram that we used above to construct the simpler biatomic structures above. First we need to place the ions and their charge states into the table. In this case, we know that sodium is a cation with a +1 charge and the phosphate ion is an anion with a -3 charge.
Note that in our table, we are treating the polyatomic ion as a single unit. We can then continue to use our cross multiplication strategy to determine how many cations and anions are needed to create an overall molecule that is neutral in charge.
Thus, we will need 3 atoms of sodium and one molecule of phosphate to complete our structure. Overall the chemical formula of sodium phosphate is written as Na3PO4. Note that the naming of the resulting molecule is done in exactly the same way as with other ionic compounds. The name of the cation comes first (using roman numerals when necessary) followed by the name of the anion (in this case phosphate).
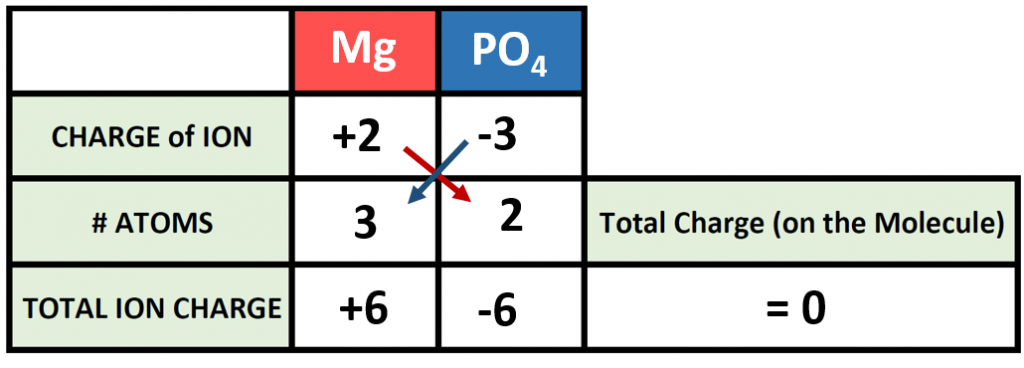
How about a more complicated example? How would we make a molecule of magnesium phosphate? Start building your molecule using the charge box diagram, noting this time that magnesium forms and Mg2+ ion.
Setting up the charge box for this compound is not more difficult than any other compound. However, one must be careful when writing out compounds that require more than one polyatomic ion within the chemical formula. In this case we need 2 phosphate ions to combine with 3 magnesium ions to form magnesium phosphate. The cation in this case is written the same, however, parentheses are needed when expressing the 2 phosphate ions, as follows:
Mg3(PO4)2
The parentheses around the phosphate ion ensure that it is clear that you need two entire PO43- ions within this complex. A structural diagram of what this molecule would look like is shown below. Note that each straight line is being used here to indicate a covalent bond within the phosphate ion. Each straight line represents two electrons (or an electron pair) that is being shared between the atoms. Covalent bonding will be described in more detail in chapter 4. For now, it is important to remember that the polyatomic ions move together as a single unit because the atoms that are sharing electrons must stay in close proximity with one another. The ionic bonds are indicated with the (+) and (-) symbols. For magnesium phosphate there are a total of 6 ionic bonds that are formed.
Another strange example is mercury (I) chloride. This one is an exception to our normal bonding rules. You would predict based on charge possibilities that mercury (I) chloride should have the chemical formula of HgCl, as the chloride ion has a charge of -1, and mercury (I) is indicated to have a charge of +1. However, in this unique case, this formula is incorrect. Mercury is unusual in that its singly ionized oxidation state, mercury(I), is found as a dimeric cation, Hg22+, where two atoms of mercury are actually covalently bonded to one another as a polyatomic ion. Each mercury atom within the bonded pair has a charge state of +1. This give the overall ion a +2 state, as shown below:
Unfortunately, this polyatomic ion does not have a unique name that distinguishes it from normal monoatomic cations. Thus, you will need to remember this unique member. The final mercury (I) chloride chemical formula needs 2 chloride ions to complete the structure, for a minimal chemical formula of Hg2Cl2.
While mercury (I) chloride is rarely found in nature, during the 18th and 19th centuries, known as calomel, it was commonly used as medicine to treat infectious diseases like syphilis and yellow fever. It was also used as a general tonic to make patients regurgitate and release their body from ‘impurities’. Calomel had extreme side effects and toxicity during its medical use causing both loss of hair and teeth. In fact, calomel was also a common ingredient in teething powders in Britain up until 1954, causing widespread mercury poisoning in the form of pink disease, which at the time had a mortality rate of 1 in 10. Once the cause of pink disease was linked with mercury toxicity, the substance was removed from these powders. In the United States, its use faded in the late 1800’s with the discovery of more effective treatments, such as the discovery of penicillin in the late 19th century by Alexander Flemming.
Abraham Lincoln and “Blue Mass”
“Blue mass,” a medication that consisted of elemental mercury with various additives, was commonly used for all kinds of complaints in the Civil War-era United States. Though mercury was a known toxin, it was a prominent feature in medical treatment for “hypochondriasis,” a condition that may have included various problems we now understand as mood disorders, along with digestive system issues. Abraham Lincoln was known to exhibit the symptoms of hypochondriasis, and he took the blue mass medication. Interestingly, he was known by friends and acquaintances to suffer from insomnia and erratic mood, and there is some evidence that he displayed additional neurological abnormalities. These are symptoms of mercury poisoning. Within the body, elemental mercury, which is uncharged, is oxidized to its mercuric form (Hg2+), which has a +2 charge. This form of mercury is devastating to many body systems, causing dysfunction that may have been responsible for Abraham Lincoln’s symptoms. His treatment may have been more harmful than the problems for which it was intended, due to medicine’s lack of understanding.
3.7 Naming Polyatomic Ions
Polyatomic ions have special names. Many of them contain oxygen and are called oxyanions. When only one oxyanion for an element exists, the ending of the primary element is given the ‘-ate’ ending. For example, the oxyanion of carbon is called carbonate (CO32-). However, when different oxyanions exist using the same element but have a different number of oxygen atoms, prefixes and suffixes are used to tell them apart. For example, if two oxyanions exist, the one with the lower number of oxygens will be given the ‘-ite’ ending and the one with more oxygens will be given the ‘-ate’ ending. Oxyanions of nitrogen and sulfur are a good example:
NO2– is called Nitrite
NO3– is called Nitrate
SO32- is called Sulfite
SO42- is called Sulfate
Sometimes there may be three or four oxyanions. In this case, the prefix ‘hypo-‘ will be used to indicate one less oxygen than ‘-ite’ form. When four oxyaions exist there is also a ‘per-‘ prefix, meaning one more oxygen that the ‘-ate’ form. The chlorine family of ions is an excellent example where these prefixes are needed.
ClO– is called hypochlorite
ClO2– is called chlorite
ClO3– is called chlorate
ClO4– is called perchlorate
Occasionally, you will see a bi– prefix. This is an older prefix, it means the compound can both take up and lose a proton (H+). IUPAC nomenclature will use hydrogen in the name, whereas the older nomenclature uses the bi-prefix. In either case, the oxyanion will have a hydrogen in it, decreasing its charge by one. For instance, there is carbonate (CO32-) and hydrogen carbonate (HCO3–). You may also see hydrogen carbonate referred to as bicarbonate.
One last prefix you may find is thio-. It means an oxygen has been replaced with a sulfur within the oxyanion. Cyanate is OCN–, and thiocyanate is SCN–.
Naming ionic compounds that contain polyatomic ions is done in exactly the same way as with other binary ionic compounds. The name of the cation comes first (using roman numerals when necessary) followed by the name of the anion.
3.8 Properties and Types of Ionic Compounds
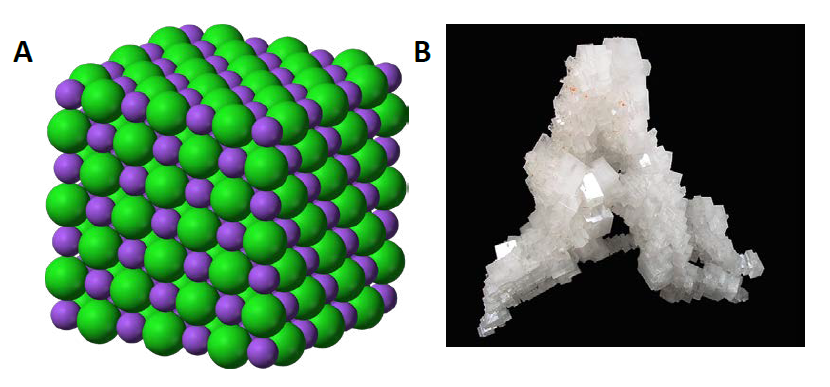
Ionic compounds are held together by the electrostatic forces created by the attraction of the positively charged cations and negatively charged anions. These can be simple ions such as the sodium (Na+) and chloride (Cl−) in sodium chloride, or polyatomic species such as the ammonium (NH4+) and carbonate (CO32-) ions in ammonium carbonate. Individual ions within an ionic compound usually have multiple nearest neighbors, so are not considered to be part of individual molecules, but instead as part of a continuous three-dimensional network or lattice, usually in a crystalline structure. Figure 4.6 shows the structure of sodium chloride (NaCl)
Figure 4.6 Crystal Lattice. (A) The crystal structure of sodium chloride, NaCl, a typical ionic compound. The purple spheres represent sodium cations, Na+, and the green spheres represent chloride anions, Cl−. (B) Halite, the mineral form of sodium chloride, forms when salty water evaportates leaving the ions behind.
Source: (A) Benjah-bmm27 (2010). (B) Lavisky, R. (2010) Both (A) and (B) Available at: https://en.wikipedia.org/wiki/Ionic_compound
Ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing hydroxide (OH−) or oxide (O2−) ions are classified as bases. All other ionic compounds without these ions are known as salts. Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids, they are most often electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
FOR A COOL VIDEO ON THE OXIDATIVE PROPERTIES OF SALTPETER, CLICK HERE!
3.9 Arrhenius Acids and Bases
H+ and OH– ions are the key players in acid-base chemistry, under the Arrhenius definitions for acids and bases. Arrhenius defined an acid as a compound that increases the concentration of hydrogen cations (H+) in aqueous solution. Many acids are simple compounds that release a hydrogen cation into solution when they dissolve and can be recognized as ionic compounds that contain H+ as the cation. Similarly, Arrhenius defined a base as a compound that increases the concentration of hydroxide ions (OH−) in aqueous solution. Many bases are ionic compounds that have the hydroxide ion as their anion, which is released when the base dissolves in water.
Arrhenius bases are named according to standard ionic nomenclature, with the strongest bases being the hydroxides of the alkali metals and the heavier alkaline earth metals. You will be expected to recognize strong bases.
Arrhenius acids have a nomenclature system that is a little more complex, since their structures can include both binary compounds as well as polyatomic anions. In naming acids from binary compounds, the prefix ‘hydro-‘ is used to represent the cation H+, and the suffix ‘-ic’ acid is used to indicate that it is an acidic form. The element name of the anion can be used directly, as is the case for H2S known as hydrosulfuric acid, or more commonly, the anion is modified by dropping the ‘-ine’, ‘-ous’ or ‘-ogen’ ending before replacing with the suffix ‘-ic acid’, as is the case for HCl which is known as hydrochloric acid, H3P which is known as hydrophosphoric acid and H3N which is known as hydronitric acid.
If an acid contains a polyatomic ion, no leading prefix is used to indicate the H+ cation. This is implied within the name. For polyatomic anions ending with the suffix ‘-ate’, the acid is named as the [anion name] + the ‘-ic acid’ suffix. For example, when the sulfate ion (SO42-) is complexed with H+ as the cation, the overall formula will be H2SO4 and the resulting acid will be named sulfuric acid. Dropping the prefix distinguishes polyatomic acids from the binary acids, in this case sulfuric acid (H2SO4) is distinguished from hydrosulfuric acid (H2S). If a polyatomic anion has the ‘-ite’ ending, the acid name will be written as the [anion name] + the ‘-ous acid’ suffix. For example HNO2 would be nitrous acid, and HNO3 would be nitric acid. The prefixes ‘hypo-‘ and ‘per-‘ are also retained in the acid nomenclature for elements that have many oxyanion states. For example the chlorine containing oxyanions can form the following acids:
HClO = hypochlorous acid
HClO2 = chlorous acid
HClO3 = chloric acid
HClO4 = perchloric acid
These are all distinguished from the binary chlorine-containing acid:
HCl = hydrochloric acid
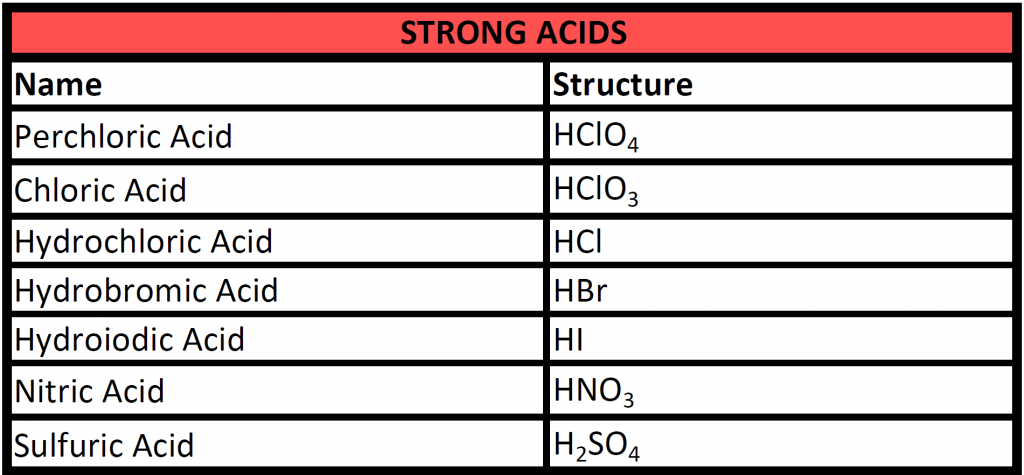
Strong acids are ones that completely dissociate into their ionic forms in solution. The following table lists common strong acids that you will need to be familiar with.
Quiz Yourself: More Practice Naming Compounds
3.10 Chapter Summary
If an atom has gained one or more electrons, it is negatively charged and is called an anion. If an atom has lost one or more electrons, it is positively charged and is called a cation. Metals generally form cations while nonmetals generally form anions. Because opposite charges attract (while like charges repel), these oppositely charged ions attract each other, forming ionic bonds. The resulting compounds are called ionic compounds. The simplest ionic compounds are binary ionic compounds or those that only contain two atoms, one acting as the cation, and one acting as the anion.
The tendency of an atom toward a configuration in which it possesses eight valence electrons is referred to as the “Octet Rule.” The term isoelectronic refers to an atom and an ion of a different atom (or two different ions) that have the same electron configuration. Cations lose electrons to become isoelectronic with the noble gas in the previous row (period) on the table. Anions gain electrons to become isoelectronic with the noble gas in the same row as the anion. The periodic table can be used to predict common ion states for the elements
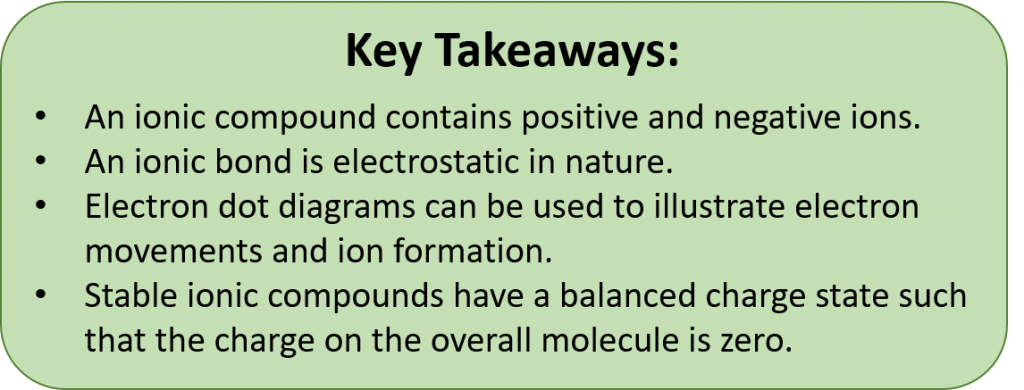
During ionic bond formation, electron dot diagrams can be used to illustrate electron movements. Stable ionic compounds have a balanced charge state such that the charge on the overall molecule is zero. When writing chemical formulas, the cation is always first and the anion is always last. Stable chemical formulas must be written so that the overall compound has a net neutral charge (ie the total positive charge = the total negative charge). Subscripts are used to show how many atoms are present within an ionic formula. Chemical formulas are always reduced to show the lowest number of each cation and anion required for a single compound to form.
Cations are named by using the element name followed by the word ‘ion’. Roman numerals are added after the element name if a cation has more than one ionic form. Anions are named by dropping the last part of the element name and replacing it with the suffix ‘-ide’ followed by the word ‘ion’. When naming an ionic compound the cation name, including roman numerals when needed, is placed first, followed by the anion name.
Polyatomic ions are ions that form from multiple atoms that are covalently bonded together. Polyatomic ions behave as a single group when participating in ionic bonding. Oxyanions are polyatomic anions that contain oxygen as one of the elemental components. If only one oxyanion exists for a specific element it is given the ‘-ate’ suffix within the name. If two oxyanions exist for a specific element, the one with fewer oxygens is given the ‘-ite’ ending and the one with more oxygens the ‘-ate’ ending. If three or four oxyanion species exist for a specific element the prefix ‘hypo-‘ and suffix ‘-ite’ are used to show one less oxygen below the ‘-ite’ anion, and the prefix ‘per-‘ and suffix ‘-ate’ are used to show one additional oxygen above the ‘-ate’ anion. Naming ionic compounds that contain polyatomic ions is done in exactly the same way as with other binary ionic compounds. The name of the cation comes first (using roman numerals when necessary) followed by the name of the anion.
Solid ionic compounds typically form a continuous three-dimensional network or lattice, usually in a crystalline structure, rather than individual molecules. Ionic compounds typically have high melting and boiling points, and are hard and brittle. As solids, they are most often electrically insulating, but when melted or dissolved they become highly conductive, because the ions are mobilized.
Using the Arrhenius definitions, ionic compounds containing hydrogen ions (H+) are classified as acids, and those containing hydroxide (OH−) or oxide (O2−) ions are classified as bases. All other ionic compounds without these ions are known as salts. Naming salts and basic ionic compounds follows standard ionic nomenclature rules. In naming acids from binary compounds, the prefix ‘hydro-‘ is used to represent the cation H+, and the suffix ‘-ic’ acid is used to indicate that it is an acidic form. If an acid contains a polyatomic ion, no leading prefix is used to indicate the H+ cation. This is implied within the name. For polyatomic anions ending with the suffix ‘-ate’, the acid is named as the [anion name] + the ‘-ic acid’ suffix. If a polyatomic anion has the ‘-ite’ ending, the acid name will be written as the [anion name] + the ‘-ous acid’ suffix. The prefixes ‘hypo-‘ and ‘per-‘ are also retained in the acid nomenclature for elements that have many oxyanion states.
3.11 References
- Ball, D. W.; Hill J. W.; Scott, R. J. The Basics of General, Organic, and Biological Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry_Textbook_Maps/Map%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)
- Poulsen, T. (2010) Introduction to Chemistry. Published under Creative Commons by-nc-sa 3.0. Available at: http://openedgroup.org/books/Chemistry.pdf
- Wikipedia. Mercury (element). Published under Creative Commons by-sa 3.0 Unported License. Available at: https://en.wikipedia.org/wiki/Mercury_(element)
- Wikipedia. St. Elmo’s fire. Published under Creative Commons by-sa 3.0 Unported License. Available at: https://en.wikipedia.org/wiki/St._Elmo’s_fire
- Bernhoft R. A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health. 2012, 2012, 1-10. Available from: https://www.hindawi.com/journals/jeph/2012/460508/abs/
- Wikipedia. Bromoperoxidase. Published under Creative Commons by-sa 3.0 Unported License. Available at: https://en.wikipedia.org/wiki/Bromoperoxidase
- Bewick, S., Parsons, R., Forsythe, T., Robinson, S., and Dupon, J. (2016) Introductory Chemistry (CK-12) LibreTexts. https://chem.libretexts.org/Textbook_Maps/Introductory_Chemistry_Textbook_Maps/Map%3A_Introductory_Chemistry_(CK-12)/08%3A_Ionic_and_Metallic_Bonding/8.03%3A_Cation_Formation
- General Chemistry/Naming Substances. (2017, March 16). Wikibooks, The Free Textbook Project. Retrieved 15:35, April 13, 2017 from https://en.wikibooks.org/w/index.php?title=General_Chemistry/Naming_Substances&oldid=3196789.
- Mercury(I) chloride. (2017, January 18). In Wikipedia, The Free Encyclopedia. Retrieved 18:21, April 14, 2017, from https://en.wikipedia.org/w/index.php?title=Mercury(I)_chloride&oldid=760689931
- Blue mass. (2016, October 2). In Wikipedia, The Free Encyclopedia. Retrieved 18:11, April 15, 2017, from https://en.wikipedia.org/w/index.php?title=Blue_mass&oldid=742175689